当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectroscopic Investigation of the Role of Water in Copper Zeolite Methane Oxidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c06010 Alexander J. Heyer 1, 2 , Jing Ma 2 , Dieter Plessers 2 , Augustin Braun 1 , Max L. Bols 2 , Hannah M. Rhoda 1 , Robert A. Schoonheydt 2 , Bert F. Sels 2 , Edward I. Solomon 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c06010 Alexander J. Heyer 1, 2 , Jing Ma 2 , Dieter Plessers 2 , Augustin Braun 1 , Max L. Bols 2 , Hannah M. Rhoda 1 , Robert A. Schoonheydt 2 , Bert F. Sels 2 , Edward I. Solomon 1, 3
Affiliation
|
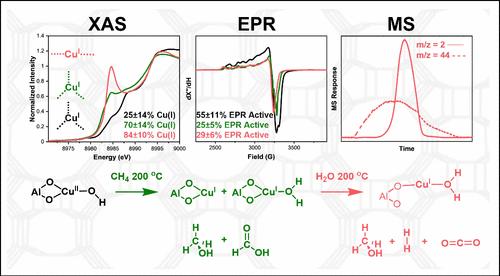
Methane is one of the most potent greenhouse gases; developing technology for its abatement is essential for combating climate change. Copper zeolites can activate methane at low temperatures and pressures, demonstrating promise for this technology. However, a barrier to industrial implementation is the inability to recycle the Cu(II) active site. Anaerobic active site regeneration has been reported for copper-loaded mordenite, where it is proposed that water oxidizes Cu(I) formed from the methane reaction, producing H2 gas as a byproduct. However, this result has been met with skepticism given the overall reaction is thermodynamically unfavorable. In this study, we use X-ray absorption and electron paramagnetic resonance spectroscopies to study the role of water in copper zeolite methane oxidation. We find that water does not oxidize Cu(I) to Cu(II) in CH4-reacted Cu-MOR. Further, using isotope label mass spectrometry, we detail an alternate source of the hydrogen byproduct. We uncover that, although water does not oxidize Cu(I), it has the potential to facilitate low temperature methane abatement through promotion of product decomposition to carbon dioxide and H2.
中文翻译:

水在铜沸石甲烷氧化中的作用的光谱研究
甲烷是最有效的温室气体之一;开发减缓气候变化的技术对于应对气候变化至关重要。铜沸石可以在低温低压下活化甲烷,展示了该技术的前景。然而,工业实施的障碍是无法回收 Cu(II) 活性位点。载铜丝光沸石的厌氧活性位点再生已有报道,其中提出水氧化甲烷反应形成的 Cu(I),产生副产物 H 2 气体。然而,由于整个反应在热力学上不利,这一结果受到了质疑。在这项研究中,我们利用 X 射线吸收和电子顺磁共振波谱研究了水在铜沸石甲烷氧化中的作用。我们发现水不会将 CH 4 反应的 Cu-MOR 中的 Cu(I) 氧化为 Cu(II)。此外,利用同位素标记质谱法,我们详细介绍了氢副产品的替代来源。我们发现,虽然水不会氧化 Cu(I),但它有可能通过促进产物分解为二氧化碳和 H 2 来促进低温甲烷减排。
更新日期:2024-07-25
中文翻译:

水在铜沸石甲烷氧化中的作用的光谱研究
甲烷是最有效的温室气体之一;开发减缓气候变化的技术对于应对气候变化至关重要。铜沸石可以在低温低压下活化甲烷,展示了该技术的前景。然而,工业实施的障碍是无法回收 Cu(II) 活性位点。载铜丝光沸石的厌氧活性位点再生已有报道,其中提出水氧化甲烷反应形成的 Cu(I),产生副产物 H 2 气体。然而,由于整个反应在热力学上不利,这一结果受到了质疑。在这项研究中,我们利用 X 射线吸收和电子顺磁共振波谱研究了水在铜沸石甲烷氧化中的作用。我们发现水不会将 CH 4 反应的 Cu-MOR 中的 Cu(I) 氧化为 Cu(II)。此外,利用同位素标记质谱法,我们详细介绍了氢副产品的替代来源。我们发现,虽然水不会氧化 Cu(I),但它有可能通过促进产物分解为二氧化碳和 H 2 来促进低温甲烷减排。












































 京公网安备 11010802027423号
京公网安备 11010802027423号