当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Reactivity of a Synthetic End-On Cobalt(II) Alkyl Persulfide Complex as a Model Platform for Thiolate Persulfidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c07276 Keyan Li 1 , Lev N. Zakharov 1 , Michael D. Pluth 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c07276 Keyan Li 1 , Lev N. Zakharov 1 , Michael D. Pluth 1
Affiliation
|
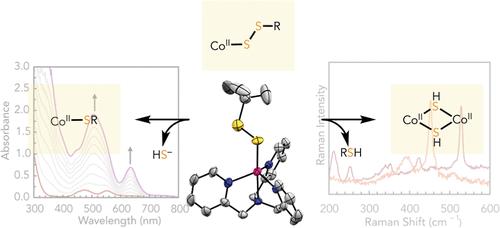
Persulfides (RSS–) are ubiquitous source of sulfides (S2–) in biology, and interactions between RSS– and bioinorganic metal centers play critical roles in biological hydrogen sulfide (H2S) biogenesis, signaling, and catabolism. Here, we report the use of contact-ion stabilized [Na(15-crown-5)][tBuSS] (1) as a simple synthon to access rare metal alkyl persulfide complexes and to investigate the reactivity of RSS– with transition metal centers to provide insights into metal thiolate persulfidation, including the fundamental difference between alkyl persulfides and alkyl thiolates. Reaction of 1 with [CoII(TPA)(OTf)]+ afforded the η1-alkyl persulfide complex [CoII(TPA)(SStBu)]+ (2), which was characterized by X-ray crystallography, UV–vis spectroscopy, and Raman spectroscopy. RSS– coordination to the Lewis acidic Co2+ center provided additional stability to the S–S bond, as evidenced by a significant increase in the Raman stretching frequency for 2 (vS–S = 522 cm–1, ΔvS–S = 66 cm–1). The effect of persulfidation on metal center redox potentials was further elucidated using cyclic voltammetry, in which the Co2+ → Co3+ oxidation potential of 2 (Ep,a = +89 mV vs SCE) is lowered by nearly 700 mV when compared to the corresponding thiolate complex [CoII(TPA)(StBu)]+ (3) (Ep,a = +818 mV vs SCE), despite persulfidation being generally seen as an oxidative post-translational modification. The reactivity of 2 toward reducing agents including PPh3, BH4–, and biologically relevant thiol reductant DTT led to different S2– output pathways, including formation of a dinuclear 2Co–2SH complex [CoII2(TPA)2(μ2-SH)2]2+(4).
中文翻译:

作为硫醇盐过硫化模型平台的合成端基钴(II)烷基过硫化物配合物的合成、表征和反应性
过硫化物 (RSS – ) 是生物学中普遍存在的硫化物 (S 2– ) 来源,RSS – 与生物无机金属中心之间的相互作用在生物氢中发挥着关键作用硫化物 (H 2 S) 生物发生、信号传导和分解代谢。在这里,我们报告了使用接触离子稳定的 [Na(15-crown-5)][ t BuSS] (1) 作为简单的合成子来获取稀有金属烷基过硫化物配合物并研究其反应性RSS – 与过渡金属中心,以提供对金属硫醇盐过硫化的见解,包括烷基过硫化物和烷基硫醇盐之间的根本区别。 1与[Co II (TPA)(OTf)] + 反应得到η 1 -烷基过硫化物络合物[Co II (TPA)(SS t Bu)] + (2),通过 X 射线晶体学、紫外可见光谱和拉曼光谱对其进行了表征。 RSS – 与路易斯酸性 Co 2+ 中心的配位为 S-S 键提供了额外的稳定性,2 的拉曼伸缩频率显着增加证明了这一点(v S–S = 522 厘米 –1 ,Δv S–S = 66 厘米 –1 )。利用循环伏安法进一步阐明了过硫化对金属中心氧化还原电位的影响,其中Co 2+ →Co 3+ 氧化电位为2(E p,a =与相应的硫醇盐配合物 [Co II (TPA)(S t Bu)] + 相比,+89 mV vs SCE) 降低了近 700 mV (3) (E p,a = +818 mV vs SCE),尽管过硫化通常被视为氧化翻译后修饰。 2 对包括 PPh 3 、BH 4 – 和生物相关硫醇还原剂 DTT 在内的还原剂的反应性导致不同的 S 2– 输出途径,包括形成双核 2Co–2SH 复合物 [Co II 2 (TPA) 2 (μ 2 -SH) 2 ] 2+ (4)。
更新日期:2024-07-25
中文翻译:

作为硫醇盐过硫化模型平台的合成端基钴(II)烷基过硫化物配合物的合成、表征和反应性
过硫化物 (RSS – ) 是生物学中普遍存在的硫化物 (S 2– ) 来源,RSS – 与生物无机金属中心之间的相互作用在生物氢中发挥着关键作用硫化物 (H 2 S) 生物发生、信号传导和分解代谢。在这里,我们报告了使用接触离子稳定的 [Na(15-crown-5)][ t BuSS] (1) 作为简单的合成子来获取稀有金属烷基过硫化物配合物并研究其反应性RSS – 与过渡金属中心,以提供对金属硫醇盐过硫化的见解,包括烷基过硫化物和烷基硫醇盐之间的根本区别。 1与[Co II (TPA)(OTf)] + 反应得到η 1 -烷基过硫化物络合物[Co II (TPA)(SS t Bu)] + (2),通过 X 射线晶体学、紫外可见光谱和拉曼光谱对其进行了表征。 RSS – 与路易斯酸性 Co 2+ 中心的配位为 S-S 键提供了额外的稳定性,2 的拉曼伸缩频率显着增加证明了这一点(v S–S = 522 厘米 –1 ,Δv S–S = 66 厘米 –1 )。利用循环伏安法进一步阐明了过硫化对金属中心氧化还原电位的影响,其中Co 2+ →Co 3+ 氧化电位为2(E p,a =与相应的硫醇盐配合物 [Co II (TPA)(S t Bu)] + 相比,+89 mV vs SCE) 降低了近 700 mV (3) (E p,a = +818 mV vs SCE),尽管过硫化通常被视为氧化翻译后修饰。 2 对包括 PPh 3 、BH 4 – 和生物相关硫醇还原剂 DTT 在内的还原剂的反应性导致不同的 S 2– 输出途径,包括形成双核 2Co–2SH 复合物 [Co II 2 (TPA) 2 (μ 2 -SH) 2 ] 2+ (4)。












































 京公网安备 11010802027423号
京公网安备 11010802027423号