当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cyclopropanation with Non-Stabilized Carbenes via Ketyl Radicals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c07388 Duong T. Ngo 1 , Jacob J. A. Garwood 1 , David A. Nagib 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c07388 Duong T. Ngo 1 , Jacob J. A. Garwood 1 , David A. Nagib 1
Affiliation
|
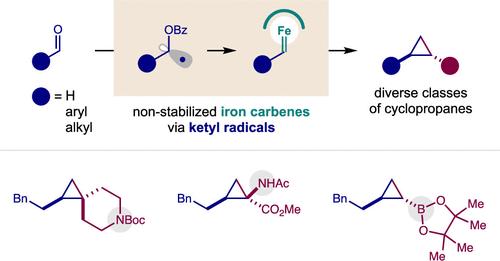
A radical mechanism enables simple and robust access to nonstabilized, alkyl iron carbenes for novel (2 + 1) cycloadditions. This Fe-catalyzed strategy employs simple, aliphatic aldehydes as carbene precursors in a practical, efficient, and stereoselective cyclopropanation. This air- and water-tolerant method permits convenient generation of iron carbenes and coupling to an exceptionally wide range of sterically and electronically diverse alkenes (nucleophilic, electrophilic, and neutral). A transient ketyl radical intermediate is key to accessing and harnessing this rare, alkyl iron carbene reactivity. Mechanistic experiments confirm the (a) intermediacy of ketyl radicals, (b) iron carbene formation by radical capture, and (c) nonconcerted nature of the (2 + 1) cycloaddition.
中文翻译:

通过烷基自由基与不稳定的卡宾进行环丙烷化
自由基机制能够简单而稳健地获得不稳定的烷基铁卡宾,以进行新型 (2 + 1) 环加成。这种铁催化策略采用简单的脂肪醛作为卡宾前体,进行实用、高效和立体选择性的环丙烷化。这种耐空气和耐水的方法可以方便地生成铁卡宾,并与范围极其广泛的空间和电子多样化的烯烃(亲核、亲电和中性)偶联。瞬态羰基自由基中间体是获得和利用这种罕见的烷基铁卡宾反应性的关键。机理实验证实了(a)羰基自由基的中介作用,(b)通过自由基捕获形成铁卡宾,以及(c)(2 + 1)环加成的非协同性质。
更新日期:2024-07-25
中文翻译:

通过烷基自由基与不稳定的卡宾进行环丙烷化
自由基机制能够简单而稳健地获得不稳定的烷基铁卡宾,以进行新型 (2 + 1) 环加成。这种铁催化策略采用简单的脂肪醛作为卡宾前体,进行实用、高效和立体选择性的环丙烷化。这种耐空气和耐水的方法可以方便地生成铁卡宾,并与范围极其广泛的空间和电子多样化的烯烃(亲核、亲电和中性)偶联。瞬态羰基自由基中间体是获得和利用这种罕见的烷基铁卡宾反应性的关键。机理实验证实了(a)羰基自由基的中介作用,(b)通过自由基捕获形成铁卡宾,以及(c)(2 + 1)环加成的非协同性质。












































 京公网安备 11010802027423号
京公网安备 11010802027423号