当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transient Covalency in Molten Uranium(III) Chloride
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-23 , DOI: 10.1021/jacs.4c05765 Dmitry S. Maltsev 1, 2 , Darren M. Driscoll 1 , Yuanpeng Zhang 3 , Joerg C. Neuefeind 3 , Benjamin Reinhart 4 , Can Agca 1 , Debmalya Ray 1 , Phillip W. Halstenberg 1, 2 , Mina Aziziha 5 , Juliano Schorne-Pinto 5 , Theodore M. Besmann 5 , Vyacheslav S. Bryantsev 1 , Sheng Dai 1, 2 , Santanu Roy 1 , Alexander S. Ivanov 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-23 , DOI: 10.1021/jacs.4c05765 Dmitry S. Maltsev 1, 2 , Darren M. Driscoll 1 , Yuanpeng Zhang 3 , Joerg C. Neuefeind 3 , Benjamin Reinhart 4 , Can Agca 1 , Debmalya Ray 1 , Phillip W. Halstenberg 1, 2 , Mina Aziziha 5 , Juliano Schorne-Pinto 5 , Theodore M. Besmann 5 , Vyacheslav S. Bryantsev 1 , Sheng Dai 1, 2 , Santanu Roy 1 , Alexander S. Ivanov 1
Affiliation
|
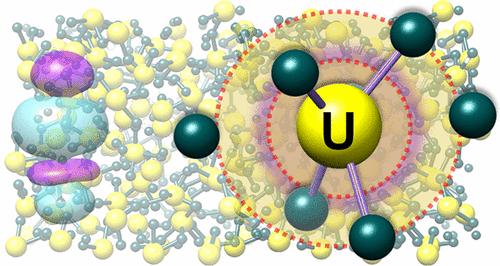
Uranium is arguably the most essential element in the actinide series, serving as a crucial component of nuclear fuels. While U is recognized for engaging the 5f orbitals in chemical bonds under normal conditions, little is known about its coordination chemistry and the nature of bonding interactions at extreme conditions of high temperature. Here we report experimental and computational evidence for the shrinkage of the average U–ligand distance in UCl3 upon the solid-to-molten phase transition, leading to the formation of a significant fraction of short, transient U–Cl bonds with the enhanced involvement of U 5f valence orbitals. These findings reveal that extreme temperatures create an unusual heterogeneous bonding environment around U(III) with distinct inner- and outer-coordination subshells.
中文翻译:

熔融氯化铀(III)中的瞬态共价
铀可以说是锕系元素中最重要的元素,是核燃料的重要组成部分。虽然 U 被认为在正常条件下与 5f 轨道形成化学键,但人们对其配位化学以及在极端高温条件下键合相互作用的性质知之甚少。在这里,我们报告了 UCl 3 中的平均 U-配体距离在固体到熔融相变时收缩的实验和计算证据,导致形成大量短的、瞬态的 U- Cl 与 U 5f 价轨道的参与增强。这些发现表明,极端温度在 U(III) 周围形成了一种不寻常的异质键合环境,具有独特的内配位和外配位亚壳。
更新日期:2024-07-25
中文翻译:

熔融氯化铀(III)中的瞬态共价
铀可以说是锕系元素中最重要的元素,是核燃料的重要组成部分。虽然 U 被认为在正常条件下与 5f 轨道形成化学键,但人们对其配位化学以及在极端高温条件下键合相互作用的性质知之甚少。在这里,我们报告了 UCl 3 中的平均 U-配体距离在固体到熔融相变时收缩的实验和计算证据,导致形成大量短的、瞬态的 U- Cl 与 U 5f 价轨道的参与增强。这些发现表明,极端温度在 U(III) 周围形成了一种不寻常的异质键合环境,具有独特的内配位和外配位亚壳。












































 京公网安备 11010802027423号
京公网安备 11010802027423号