当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery, Optimization, and Biological Evaluation of Novel Pyrazol-5-yl-phenoxybenzamide Derivatives as Potent Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-24 , DOI: 10.1021/acs.jafc.4c02685 Dan Xu 1, 2 , Guo-Tai Lin 1 , Jia-Chuan Huang 1 , Jian Sun 3 , Wei Wang 4 , Xili Liu 1 , Gong Xu 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-24 , DOI: 10.1021/acs.jafc.4c02685 Dan Xu 1, 2 , Guo-Tai Lin 1 , Jia-Chuan Huang 1 , Jian Sun 3 , Wei Wang 4 , Xili Liu 1 , Gong Xu 1, 2
Affiliation
|
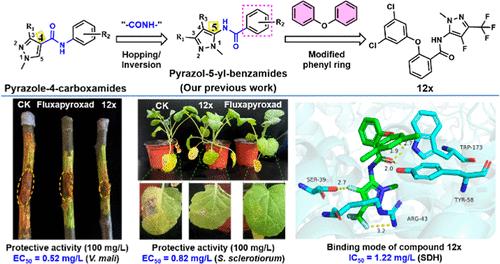
The diphenyl ether molecular pharmacophore has played a significant role in the development of fungicidal compounds. In this study, a variety of pyrazol-5-yl-phenoxybenzamide derivatives were synthesized and evaluated for their potential to act as succinate dehydrogenase inhibitors (SDHIs). The bioassay results indicate certain compounds to display a remarkable and broad-spectrum in their antifungal activities. Notably, compound 12x exhibited significant in vitro activities against Valsa mali, Gaeumannomyces graminis, and Botrytis cinerea, with EC50 values of 0.52, 1.46, and 3.42 mg/L, respectively. These values were lower or comparable to those of Fluxapyroxad (EC50 = 12.5, 1.93, and 8.33 mg/L, respectively). Additionally, compound 12x showed promising antifungal activities against Sclerotinia sclerotiorum (EC50 = 0.82 mg/L) and Rhizoctonia solani (EC50 = 1.86 mg/L), albeit lower than Fluxapyroxad (EC50 = 0.23 and 0.62 mg/L). Further in vivo experiments demonstrated compound 12x to possess effective protective antifungal activities against V. mali and S. sclerotiorum at a concentration of 100 mg/L, with inhibition rates of 66.7 and 89.3%, respectively. In comparison, Fluxapyroxad showed inhibition rates of 29.2 and 96.4% against V. mali and S. sclerotiorum, respectively. Molecular docking analysis revealed that compound 12x interacts with SDH through hydrogen bonding, π-cation, and π–π interactions, providing insights into the probable mechanism of action. Furthermore, compound 12x exhibited greater binding energy and SDH enzyme inhibitory activity than Fluxapyroxad (ΔGcal = −46.8 kcal/mol, IC50 = 1.22 mg/L, compared to ΔGcal = −41.1 kcal/mol, IC50 = 8.32 mg/L). Collectively, our results suggest that compound 12x could serve as a promising fungicidal lead compound for the development of more potent SDHIs for crop protection.
中文翻译:

作为有效琥珀酸脱氢酶抑制剂的新型吡唑-5-基-苯氧基苯甲酰胺衍生物的发现、优化和生物学评价
二苯醚分子药效团在杀菌化合物的开发中发挥了重要作用。在这项研究中,合成了多种吡唑-5-基-苯氧基苯甲酰胺衍生物,并评估了它们作为琥珀酸脱氢酶抑制剂(SDHI)的潜力。生物测定结果表明某些化合物表现出显着且广谱的抗真菌活性。值得注意的是,化合物12x对Valsa mali 、 Gaeumannomyces graminis和Botrytis cinerea表现出显着的体外活性,EC 50值分别为0.52、1.46和3.42 mg/L。这些值低于或与Fluxapyroxad相当(EC 50分别 = 12.5、1.93 和 8.33 mg/L)。此外,化合物12x对核盘菌(EC 50 = 0.82 mg/L) 和立枯丝核菌(EC 50 = 1.86 mg/L) 显示出良好的抗真菌活性,尽管低于Fluxapyroxad (EC 50 = 0.23 和 0.62 mg/L)。进一步的体内实验表明,化合物12x在浓度为100 mg/L时对苹果弧菌和核盘菌具有有效的保护性抗真菌活性,抑制率分别为66.7%和89.3%。相比之下, Fluxapyroxad对苹果弧菌和核盘菌的抑制率分别为 29.2% 和 96.4%。分子对接分析表明,化合物12x通过氢键、π-阳离子和 π-π 相互作用与 SDH 相互作用,为可能的作用机制提供了见解。 此外,化合物12x表现出比Fluxapyroxad更高的结合能和 SDH 酶抑制活性(Δ G cal = -46.8 kcal/mol,IC 50 = 1.22 mg/L,相比之下,Δ G cal = -41.1 kcal/mol,IC 50 = 8.32毫克/升)。总的来说,我们的结果表明化合物12x可以作为一种有前途的杀菌先导化合物,用于开发更有效的作物保护 SDHI。
更新日期:2024-07-24
中文翻译:

作为有效琥珀酸脱氢酶抑制剂的新型吡唑-5-基-苯氧基苯甲酰胺衍生物的发现、优化和生物学评价
二苯醚分子药效团在杀菌化合物的开发中发挥了重要作用。在这项研究中,合成了多种吡唑-5-基-苯氧基苯甲酰胺衍生物,并评估了它们作为琥珀酸脱氢酶抑制剂(SDHI)的潜力。生物测定结果表明某些化合物表现出显着且广谱的抗真菌活性。值得注意的是,化合物12x对Valsa mali 、 Gaeumannomyces graminis和Botrytis cinerea表现出显着的体外活性,EC 50值分别为0.52、1.46和3.42 mg/L。这些值低于或与Fluxapyroxad相当(EC 50分别 = 12.5、1.93 和 8.33 mg/L)。此外,化合物12x对核盘菌(EC 50 = 0.82 mg/L) 和立枯丝核菌(EC 50 = 1.86 mg/L) 显示出良好的抗真菌活性,尽管低于Fluxapyroxad (EC 50 = 0.23 和 0.62 mg/L)。进一步的体内实验表明,化合物12x在浓度为100 mg/L时对苹果弧菌和核盘菌具有有效的保护性抗真菌活性,抑制率分别为66.7%和89.3%。相比之下, Fluxapyroxad对苹果弧菌和核盘菌的抑制率分别为 29.2% 和 96.4%。分子对接分析表明,化合物12x通过氢键、π-阳离子和 π-π 相互作用与 SDH 相互作用,为可能的作用机制提供了见解。 此外,化合物12x表现出比Fluxapyroxad更高的结合能和 SDH 酶抑制活性(Δ G cal = -46.8 kcal/mol,IC 50 = 1.22 mg/L,相比之下,Δ G cal = -41.1 kcal/mol,IC 50 = 8.32毫克/升)。总的来说,我们的结果表明化合物12x可以作为一种有前途的杀菌先导化合物,用于开发更有效的作物保护 SDHI。











































 京公网安备 11010802027423号
京公网安备 11010802027423号