当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Mechanism of the β3AR Agonist Activity of a β-Blocker
ChemPlusChem ( IF 3.0 ) Pub Date : 2024-07-24 , DOI: 10.1002/cplu.202400288 Shuang Zheng 1 , Shuhao Zhang 1 , Shengjie Dai 2 , Kai Chen 2 , Kaixuan Gao 1 , Xiaoou Sun 1 , Bin Lin 2 , Xiangyu Liu 3
ChemPlusChem ( IF 3.0 ) Pub Date : 2024-07-24 , DOI: 10.1002/cplu.202400288 Shuang Zheng 1 , Shuhao Zhang 1 , Shengjie Dai 2 , Kai Chen 2 , Kaixuan Gao 1 , Xiaoou Sun 1 , Bin Lin 2 , Xiangyu Liu 3
Affiliation
|
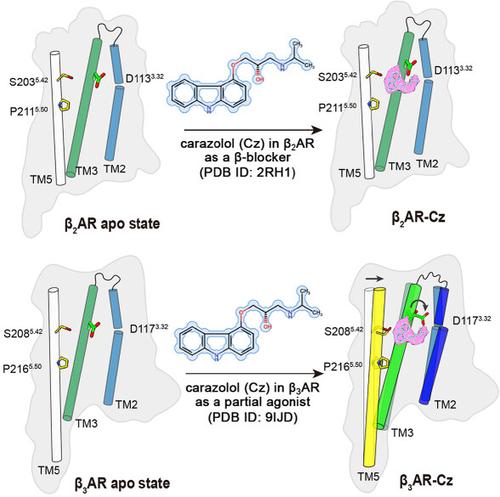
The structure of human β3AR in complex with carazolol as a β3AR partial agonist is determined (PDB ID: 9IJD, this study), which is also a high-affinity β-blocker (PDB ID: 2RH1, Cherezov et al, Science, 2007). A plausible mechanism is proposed based on structure comparison, mutagenesis studies and molecular dynamics simulations.
中文翻译:

β阻滞剂的 β3AR 激动剂活性的分子机制
确定了人 β3AR 与卡拉唑醇作为 β3AR 部分激动剂的复合物的结构 (PDB ID:9IJD,本研究),它也是一种高亲和力β阻滞剂 (PDB ID:2RH1,Cherezov et al, Science, 2007)。基于结构比较、诱变研究和分子动力学模拟,提出了一种合理的机制。
更新日期:2024-07-24
中文翻译:

β阻滞剂的 β3AR 激动剂活性的分子机制
确定了人 β3AR 与卡拉唑醇作为 β3AR 部分激动剂的复合物的结构 (PDB ID:9IJD,本研究),它也是一种高亲和力β阻滞剂 (PDB ID:2RH1,Cherezov et al, Science, 2007)。基于结构比较、诱变研究和分子动力学模拟,提出了一种合理的机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号