当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fit-for-Purpose Synthesis of a KRASG12C Covalent Inhibitor, via a Diastereoselective Hayashi Arylation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.oprd.4c00211 Carmela Molinaro 1 , Nicholas Wong 1 , Nicholas A. White 1 , Lauren E. Sirois 1 , Raphael Bigler 2 , Quentin P. Bindschaedler 2 , Steven Do 3 , Sushant Malhotra 3 , Francis Gosselin 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.oprd.4c00211 Carmela Molinaro 1 , Nicholas Wong 1 , Nicholas A. White 1 , Lauren E. Sirois 1 , Raphael Bigler 2 , Quentin P. Bindschaedler 2 , Steven Do 3 , Sushant Malhotra 3 , Francis Gosselin 1
Affiliation
|
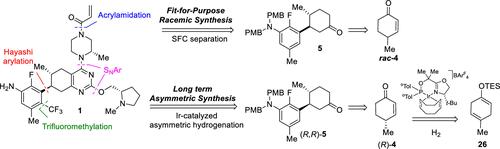
An enabling, fit-for-purpose synthesis of stereochemically pure KRASG12C covalent inhibitor 1, a potential new treatment for cancer, is described. The synthetic route provided 1 in 13 steps from commercially available 2-fluoro-5-methylaniline (2), tert-butyl (S)-3-methylpiperazine-1-carboxylate (8) and (S)-(1-methylpyrrolidin-2-yl)methanol (10). A key transformation in this sequence was the diastereoselective 1,4-addition of an aryl boronate derived from 2 with rac-4-methylcyclohex-2-en-1-one (rac-4) in a Hayashi arylation that sets two relative stereocenters of the target molecule. This in turn inspired the development of an improved synthesis of (R)-4-methylcyclohex-2-en-1-one ((R)-4) via optimized methodology for the asymmetric monohydrogenation of 1,4-dienes, thus setting the stage for a fully asymmetric synthesis of inhibitor 1.
中文翻译:

通过非对映选择性 Hayashi 芳基化合成 KRASG12C 共价抑制剂
描述了一种可行的、适合目的的立体化学纯 KRAS G12C 共价抑制剂 1 的合成,这是一种潜在的癌症新疗法。该合成路线由市售2-氟-5-甲基苯胺(2)、(S)-3-甲基哌嗪-1-甲酸叔丁酯(8)和(S)-(1-甲基吡咯烷-2)组成,共13步-基)甲醇(10)。该序列中的一个关键转化是由 2 衍生的芳基硼酸酯与 rac-4-甲基环己-2-en-1-one (rac-4) 在 Hayashi 芳基化中进行非对映选择性 1,4-加成,设置了两个相对立体中心目标分子。这反过来又激发了通过 1,4-二烯不对称单氢化的优化方法改进 (R)-4-甲基环己-2-en-1-酮 ((R)-4) 合成的开发,从而设定了抑制剂1的完全不对称合成阶段。
更新日期:2024-07-24
中文翻译:

通过非对映选择性 Hayashi 芳基化合成 KRASG12C 共价抑制剂
描述了一种可行的、适合目的的立体化学纯 KRAS G12C 共价抑制剂 1 的合成,这是一种潜在的癌症新疗法。该合成路线由市售2-氟-5-甲基苯胺(2)、(S)-3-甲基哌嗪-1-甲酸叔丁酯(8)和(S)-(1-甲基吡咯烷-2)组成,共13步-基)甲醇(10)。该序列中的一个关键转化是由 2 衍生的芳基硼酸酯与 rac-4-甲基环己-2-en-1-one (rac-4) 在 Hayashi 芳基化中进行非对映选择性 1,4-加成,设置了两个相对立体中心目标分子。这反过来又激发了通过 1,4-二烯不对称单氢化的优化方法改进 (R)-4-甲基环己-2-en-1-酮 ((R)-4) 合成的开发,从而设定了抑制剂1的完全不对称合成阶段。












































 京公网安备 11010802027423号
京公网安备 11010802027423号