当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cascade Oxypalladation/1,3-Palladium Shift to Access Cyclopentene-Fused Isocoumarins
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.orglett.4c01997 Vaibhav B. Patil 1, 2 , G. Raghu Ramudu 1, 2 , Rambabu Chegondi 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.orglett.4c01997 Vaibhav B. Patil 1, 2 , G. Raghu Ramudu 1, 2 , Rambabu Chegondi 1, 2
Affiliation
|
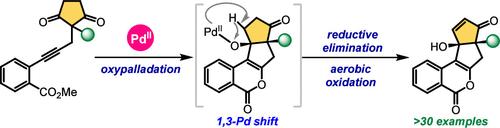
Fused isocoumarins are frequently found in several natural products and pharmaceuticals. Herein, a cascade annulation of 2-alkynylbenzoate-tethered cyclic 1,3-diones via sequential trans-oxypalladation, carbonyl insertion, 1,3-Pd shift, and β-hydride elimination is reported. This method provides efficient access to highly diastereoselective tetracyclic cyclopentene-fused isocoumarins containing two contiguous quaternary stereocenters. A plausible reaction mechanism is proposed on the basis of mechanistic studies, including deuterium labeling experiments. Studies toward enantioselective synthesis using a chiral Bpy ligand gave encouraging initial results.
中文翻译:

级联氧钯化/1,3-钯转移以获得环戊烯稠合异香豆素
稠合异香豆素常见于多种天然产物和药物中。在此,报道了通过连续的反氧基钯化、羰基插入、1,3-Pd 移位和 β-氢化物消除,2-炔基苯甲酸酯系链的环状 1,3-二酮的级联环化。该方法提供了高效获得含有两个连续四元立体中心的高度非对映选择性四环环戊烯稠合异香豆素。基于机制研究(包括氘标记实验)提出了一种合理的反应机制。使用手性 Bpy 配体进行对映选择性合成的研究给出了令人鼓舞的初步结果。
更新日期:2024-07-24
中文翻译:

级联氧钯化/1,3-钯转移以获得环戊烯稠合异香豆素
稠合异香豆素常见于多种天然产物和药物中。在此,报道了通过连续的反氧基钯化、羰基插入、1,3-Pd 移位和 β-氢化物消除,2-炔基苯甲酸酯系链的环状 1,3-二酮的级联环化。该方法提供了高效获得含有两个连续四元立体中心的高度非对映选择性四环环戊烯稠合异香豆素。基于机制研究(包括氘标记实验)提出了一种合理的反应机制。使用手性 Bpy 配体进行对映选择性合成的研究给出了令人鼓舞的初步结果。












































 京公网安备 11010802027423号
京公网安备 11010802027423号