当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Achiral Counteranion-Induced Reversal of Enantioselectivity in Ni(II)-Catalyzed Friedel–Crafts Alkylation/Annulation of 2-Naphthols
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.orglett.4c02156 Chen-Ying Hou 1 , Chun Yang 1 , Yin Tian 2 , Ming-Sheng Xie 1 , Hai-Ming Guo 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.orglett.4c02156 Chen-Ying Hou 1 , Chun Yang 1 , Yin Tian 2 , Ming-Sheng Xie 1 , Hai-Ming Guo 1
Affiliation
|
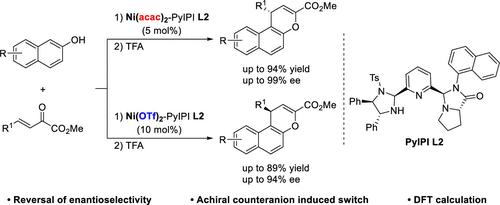
An achiral counteranion-induced reversal of enantioselectivity in Ni(II)-catalyzed Friedel–Crafts alkylation/annulation of 2-naphthols with β,γ-unsaturated α-keto esters was achieved. Using imidazolidine pyrroloimidazolone pyridine as the ligand and Ni(acac)2 as the Lewis acid, diverse naphthopyran derivatives were obtained in good yields (up to 94% yield) and high enantioselectivities (up to 99% ee). In the presence of Ni(OTf)2 as the Lewis acid, a series of chiral naphthopyran derivatives were obtained in good yields and with a controlled switch in stereoselectivity. DFT calculations reveal that the achiral counteranions regulate H-bonding interactions between counteranions with the N–H of the ligand and the O–H of 2-naphthol.
中文翻译:

Ni(II) 催化的 2-萘酚傅克烷基化/成环反应中非手性抗衡阴离子诱导的对映选择性逆转
在 Ni(II) 催化的 2-萘酚与 β,γ-不饱和 α-酮酯的弗里德尔-克来福特烷基化/成环反应中,实现了非手性抗衡阴离子诱导的对映选择性逆转。以咪唑烷吡咯并咪唑啉酮吡啶为配体,Ni(acac) 2为Lewis酸,以良好的产率(高达94%的产率)和高的对映选择性(高达99%ee)获得了多种萘并吡喃衍生物。在Ni(OTf) 2作为路易斯酸存在下,以良好的产率获得了一系列手性萘并吡喃衍生物,并具有可控的立体选择性转换。 DFT 计算表明,非手性抗衡阴离子调节抗衡阴离子与配体的 N-H 和 2-萘酚的 O-H 之间的 H 键相互作用。
更新日期:2024-07-23
中文翻译:

Ni(II) 催化的 2-萘酚傅克烷基化/成环反应中非手性抗衡阴离子诱导的对映选择性逆转
在 Ni(II) 催化的 2-萘酚与 β,γ-不饱和 α-酮酯的弗里德尔-克来福特烷基化/成环反应中,实现了非手性抗衡阴离子诱导的对映选择性逆转。以咪唑烷吡咯并咪唑啉酮吡啶为配体,Ni(acac) 2为Lewis酸,以良好的产率(高达94%的产率)和高的对映选择性(高达99%ee)获得了多种萘并吡喃衍生物。在Ni(OTf) 2作为路易斯酸存在下,以良好的产率获得了一系列手性萘并吡喃衍生物,并具有可控的立体选择性转换。 DFT 计算表明,非手性抗衡阴离子调节抗衡阴离子与配体的 N-H 和 2-萘酚的 O-H 之间的 H 键相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号