当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling Electrochemical Vacancy Regeneration in Single-Walled Carbon Nanotubes
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-07-24 , DOI: 10.1021/acs.jpclett.4c01293 Jana Jelušić 1, 2 , Jan Paul Menzel 1, 2 , Quentin C. Bertrand 1 , Robert H. Crabtree 1, 2 , Hailiang Wang 1, 2 , Gary W. Brudvig 1, 2 , Victor S. Batista 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-07-24 , DOI: 10.1021/acs.jpclett.4c01293 Jana Jelušić 1, 2 , Jan Paul Menzel 1, 2 , Quentin C. Bertrand 1 , Robert H. Crabtree 1, 2 , Hailiang Wang 1, 2 , Gary W. Brudvig 1, 2 , Victor S. Batista 1, 2
Affiliation
|
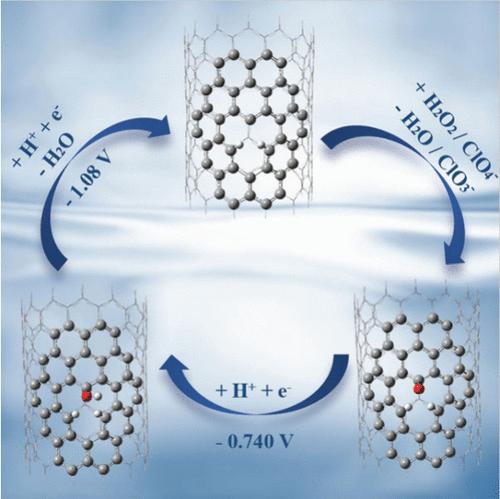
Synthesis-induced defects in single-walled carbon nanotubes (SWCNTs) enable diverse catalytic reactions, but the nature of catalytic intermediates and how active species regeneration occurs are unclear. Using a quantum mechanics/molecular mechanics (QM/MM) hybrid methodology based on density functional theory (DFT) and a classical force-field, we explore the reactivity and electrochemical regeneration of a vacancy defect in a zigzag SWCNT. Our findings indicate that hydrolysis of the defect forms a ketone group on one carbon atom and C–H bonds on two adjacent carbons. Applying an electrochemical potential of ESHE = −0.740 V triggers a proton-coupled electron transfer (PCET), converting the ketone to a hydroxyl group. Further reduction at ESHE = −1.08 V induces another PCET, expelling the hydroxyl as water and forming an active carbon with carbene character that can react with hydrogen peroxide and perchlorate. The hydrogen atoms on neighboring carbons prevent further water dissociation, maintaining the catalytic vacancy.
中文翻译:

单壁碳纳米管中电化学空位再生的模拟
单壁碳纳米管(SWCNT)中合成引起的缺陷能够实现多种催化反应,但催化中间体的性质以及活性物质再生如何发生尚不清楚。使用基于密度泛函理论 (DFT) 和经典力场的量子力学/分子力学 (QM/MM) 混合方法,我们探索了锯齿形 SWCNT 中空位缺陷的反应性和电化学再生。我们的研究结果表明,缺陷的水解在一个碳原子上形成酮基,并在两个相邻碳上形成 C-H 键。施加 E SHE = -0.740 V 的电化学势会触发质子耦合电子转移 (PCET),将酮转化为羟基。在 E SHE = -1.08 V 处进一步还原会产生另一个 PCET,以水的形式排出羟基并形成具有卡宾特性的活性炭,可与过氧化氢和高氯酸盐反应。相邻碳上的氢原子可防止水进一步解离,从而保持催化空位。
更新日期:2024-07-25
中文翻译:

单壁碳纳米管中电化学空位再生的模拟
单壁碳纳米管(SWCNT)中合成引起的缺陷能够实现多种催化反应,但催化中间体的性质以及活性物质再生如何发生尚不清楚。使用基于密度泛函理论 (DFT) 和经典力场的量子力学/分子力学 (QM/MM) 混合方法,我们探索了锯齿形 SWCNT 中空位缺陷的反应性和电化学再生。我们的研究结果表明,缺陷的水解在一个碳原子上形成酮基,并在两个相邻碳上形成 C-H 键。施加 E SHE = -0.740 V 的电化学势会触发质子耦合电子转移 (PCET),将酮转化为羟基。在 E SHE = -1.08 V 处进一步还原会产生另一个 PCET,以水的形式排出羟基并形成具有卡宾特性的活性炭,可与过氧化氢和高氯酸盐反应。相邻碳上的氢原子可防止水进一步解离,从而保持催化空位。












































 京公网安备 11010802027423号
京公网安备 11010802027423号