当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Leveraging ICH M7 Control Options 3 and 4: Discussion and Clarification Using Industrial Case Studies
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.oprd.4c00207 Michael W. Urquhart 1 , Michael J. Burns 2 , Hugh F. Clark 1 , Jean-Philippe Crochard 3 , Olivier Dirat 4 , Stefan Hildbrand 3 , Christian Moessner 3 , David D. Pascoe 1 , Alastair J. Roberts 1 , Andrew Teasdale 5 , Oliver R. Thiel 6
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.oprd.4c00207 Michael W. Urquhart 1 , Michael J. Burns 2 , Hugh F. Clark 1 , Jean-Philippe Crochard 3 , Olivier Dirat 4 , Stefan Hildbrand 3 , Christian Moessner 3 , David D. Pascoe 1 , Alastair J. Roberts 1 , Andrew Teasdale 5 , Oliver R. Thiel 6
Affiliation
|
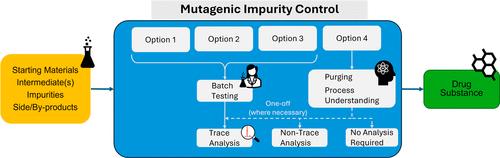
The assessment and control of potential mutagenic impurities (PMIs) within pharmaceutical products are managed in accordance with the ICH M7 guideline, “Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk”. This guideline highlights four control options, which can be used to give assurance of control of PMIs to below a level of concern for the intended patient. These controls vary from testing to confirm levels within the active pharmaceutical ingredient or product (ICH M7 Option 1 Control) to a control strategy that relies on process controls and scientific principles in lieu of analytical testing, which can include considerations of fate and purge (measured or predicted) (Option 4). This work provides clarity on when additional data or information can be useful to give greater support to an ICH M7 Option 4 control, illustrated through a series of case studies. These case studies range from examples that have been approved by health authorities for clinical applications and marketing authorizations to those that have not been submitted to regulators at this time. Sound data and scientific rationales are required to support the ICH M7 Option 4 control strategies. These supportive elements can be challenged by regulators, leading to a non-acceptance for the proposed control strategy and a commitment to routine testing (ICH M7 control Options 1, 2, or 3). This study provides an industry perspective of Option 4, approaches with an emphasis on the types of supportive datasets and scientific principles that can be used while remaining aligned with the intention of the ICH M7 guideline, and elaborates on the differences between the different control options.
中文翻译:

利用 ICH M7 控制选项 3 和 4:使用工业案例研究进行讨论和澄清
药品中潜在致突变杂质 (PMI) 的评估和控制按照 ICH M7 指南“评估和控制药品中 DNA 反应性(致突变)杂质以限制潜在致癌风险”进行管理。本指南强调了四种控制选项,可用于确保将 PMI 控制在目标患者关注的水平以下。这些控或预测)(选项 4)。这项工作通过一系列案例研究说明了何时可以使用额外的数据或信息来为 ICH M7 选项 4 控制提供更大的支持。这些案例研究的范围从已被卫生当局批准用于临床应用和营销授权的示例到目前尚未提交给监管机构的示例。需要可靠的数据和科学原理来支持 ICH M7 选项 4 控制策略。这些支持性要素可能会受到监管机构的质疑,导致不接受拟议的控制策略并承诺进行常规测试(ICH M7 控制选项 1、2 或 3)。本研究提供了选项 4 的行业视角,重点关注可使用的支持数据集类型和科学原理,同时与 ICH M7 指南的意图保持一致,并详细阐述了不同控制选项之间的差异。
更新日期:2024-07-25
中文翻译:

利用 ICH M7 控制选项 3 和 4:使用工业案例研究进行讨论和澄清
药品中潜在致突变杂质 (PMI) 的评估和控制按照 ICH M7 指南“评估和控制药品中 DNA 反应性(致突变)杂质以限制潜在致癌风险”进行管理。本指南强调了四种控制选项,可用于确保将 PMI 控制在目标患者关注的水平以下。这些控或预测)(选项 4)。这项工作通过一系列案例研究说明了何时可以使用额外的数据或信息来为 ICH M7 选项 4 控制提供更大的支持。这些案例研究的范围从已被卫生当局批准用于临床应用和营销授权的示例到目前尚未提交给监管机构的示例。需要可靠的数据和科学原理来支持 ICH M7 选项 4 控制策略。这些支持性要素可能会受到监管机构的质疑,导致不接受拟议的控制策略并承诺进行常规测试(ICH M7 控制选项 1、2 或 3)。本研究提供了选项 4 的行业视角,重点关注可使用的支持数据集类型和科学原理,同时与 ICH M7 指南的意图保持一致,并详细阐述了不同控制选项之间的差异。












































 京公网安备 11010802027423号
京公网安备 11010802027423号