当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acyl Fluorides and Chlorides: Synthesis of Aryl Halides via Reductive Elimination of the C–X (X = I, Br, and Cl) Bond and Mechanistic Implications
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-25 , DOI: 10.1021/acscatal.4c03731 Tian Tian 1 , Myuto Kashihara 2 , Weidan Yan 1 , Yasushi Nishihara 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-25 , DOI: 10.1021/acscatal.4c03731 Tian Tian 1 , Myuto Kashihara 2 , Weidan Yan 1 , Yasushi Nishihara 2
Affiliation
|
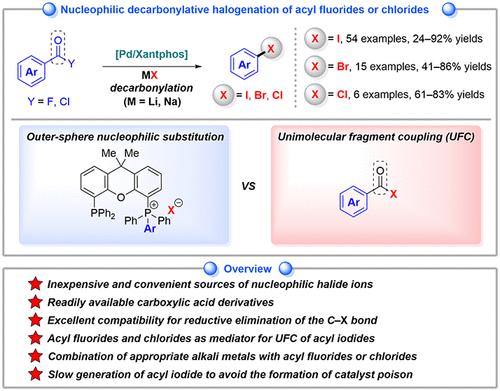
Aryl halides are widely recognized as crucial and versatile feedstocks for organic synthesis. However, in palladium-catalyzed reactions, while oxidative addition of carbon–halogen bonds is thermodynamically favorable, the reverse reaction─reductive elimination with the formation of carbon–halogen bonds─poses a significant challenge. As part of conducting a series of decarbonylative transformations of acyl halides, we developed a decarbonylative nucleophilic halogenation of acyl fluorides and chlorides through Pd-mediated reductive elimination of the C–X bond. These reactions enable the synthesis of aryl iodides, bromides, and chlorides using alkali metal halides. Regarding the reaction mechanism, the Xantphos ligand emerges as a crucial factor in promoting reductive elimination, leading to the formation of a stable Pd(0) intermediate and an oxidative adduct trans-(Xantphos)Pd(ArCO)X. Two proposed mechanisms involve Xantphos-promoted outer-sphere nucleophilic substitution and direct transhalogenation between acyl halides and alkali metal halides. In the latter mechanism, acyl fluorides or acyl chlorides react with alkali metal halides to form the corresponding acyl iodides or acyl bromides in situ and under mild conditions through decarbonylation, yielding the desired aryl halides via unimolecular fragment coupling. Importantly, it is evident that controlling the rate of acyl halide formation through the appropriate combination of substrates and alkali metal halides is crucial for the success of this reaction. Indeed, we found that the gradual formation of acyl iodide is pivotal in managing the undesired generation of I2, a known catalyst poison. This observation enables us to fine-tune reaction conditions, thereby improving the selectivity of the desired transformation. As a result, we achieve enhanced yields of the final products and establish more sustainable and robust catalytic processes. This advancement not only boosts the applicability and reliability of our synthetic methodology but also underscores the potential for broader adoption in organic synthesis.
中文翻译:

钯催化酰基氟化物和氯化物的脱羰亲核卤化:通过 C–X(X = I、Br 和 Cl)键的还原消除合成芳基卤化物及其机理意义
芳基卤化物被广泛认为是有机合成的重要且通用的原料。然而,在钯催化的反应中,虽然碳-卤键的氧化加成在热力学上是有利的,但逆反应——形成碳-卤键的还原消除——却提出了重大挑战。作为进行一系列酰基卤脱羰转化的一部分,我们通过 Pd 介导的 C-X 键还原消除,开发了酰基氟化物和氯化物的脱羰亲核卤化。这些反应使得能够使用碱金属卤化物合成芳基碘化物、芳基溴化物和氯化物。关于反应机理,Xantphos配体成为促进还原消除的关键因素,导致稳定的Pd(0)中间体和氧化加合物反式-(Xantphos)Pd(ArCO)X的形成。两种提出的机制涉及 Xantphos 促进的外层亲核取代以及酰基卤和碱金属卤化物之间的直接转卤化。在后一种机理中,酰氟或酰氯与碱金属卤化物反应,在温和条件下通过脱羰作用原位形成相应的酰碘或酰溴,通过单分子片段偶联产生所需的芳基卤。重要的是,很明显,通过底物和碱金属卤化物的适当组合来控制酰卤形成的速率对于该反应的成功至关重要。事实上,我们发现酰基碘的逐渐形成对于控制 I 2 (一种已知的催化剂毒物)的不良生成至关重要。 这一观察结果使我们能够微调反应条件,从而提高所需转化的选择性。因此,我们提高了最终产品的产量,并建立了更可持续、更稳健的催化工艺。这一进步不仅提高了我们的合成方法的适用性和可靠性,而且强调了在有机合成中更广泛采用的潜力。
更新日期:2024-07-25
中文翻译:

钯催化酰基氟化物和氯化物的脱羰亲核卤化:通过 C–X(X = I、Br 和 Cl)键的还原消除合成芳基卤化物及其机理意义
芳基卤化物被广泛认为是有机合成的重要且通用的原料。然而,在钯催化的反应中,虽然碳-卤键的氧化加成在热力学上是有利的,但逆反应——形成碳-卤键的还原消除——却提出了重大挑战。作为进行一系列酰基卤脱羰转化的一部分,我们通过 Pd 介导的 C-X 键还原消除,开发了酰基氟化物和氯化物的脱羰亲核卤化。这些反应使得能够使用碱金属卤化物合成芳基碘化物、芳基溴化物和氯化物。关于反应机理,Xantphos配体成为促进还原消除的关键因素,导致稳定的Pd(0)中间体和氧化加合物反式-(Xantphos)Pd(ArCO)X的形成。两种提出的机制涉及 Xantphos 促进的外层亲核取代以及酰基卤和碱金属卤化物之间的直接转卤化。在后一种机理中,酰氟或酰氯与碱金属卤化物反应,在温和条件下通过脱羰作用原位形成相应的酰碘或酰溴,通过单分子片段偶联产生所需的芳基卤。重要的是,很明显,通过底物和碱金属卤化物的适当组合来控制酰卤形成的速率对于该反应的成功至关重要。事实上,我们发现酰基碘的逐渐形成对于控制 I 2 (一种已知的催化剂毒物)的不良生成至关重要。 这一观察结果使我们能够微调反应条件,从而提高所需转化的选择性。因此,我们提高了最终产品的产量,并建立了更可持续、更稳健的催化工艺。这一进步不仅提高了我们的合成方法的适用性和可靠性,而且强调了在有机合成中更广泛采用的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号