当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Observing Chemical and Morphological Changes in a Cu@TiOx Core@Shell Catalyst: Impact of Reversible Metal-Oxide Interactions on CO2 Activation and Hydrogenation
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-24 , DOI: 10.1021/acscatal.4c02694 Kaixi Deng 1, 2 , Xiaobo Chen 3 , Jorge Moncada 2 , Kenna L. Salvatore 1 , Ning Rui 2 , Wenqian Xu 4 , Shuting Xiang 5 , Nebojsa Marinkovic 6 , Anatoly I. Frenkel 2, 5 , Guangwen Zhou 3 , Stanislaus S. Wong 1 , José A. Rodriguez 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-24 , DOI: 10.1021/acscatal.4c02694 Kaixi Deng 1, 2 , Xiaobo Chen 3 , Jorge Moncada 2 , Kenna L. Salvatore 1 , Ning Rui 2 , Wenqian Xu 4 , Shuting Xiang 5 , Nebojsa Marinkovic 6 , Anatoly I. Frenkel 2, 5 , Guangwen Zhou 3 , Stanislaus S. Wong 1 , José A. Rodriguez 1, 2
Affiliation
|
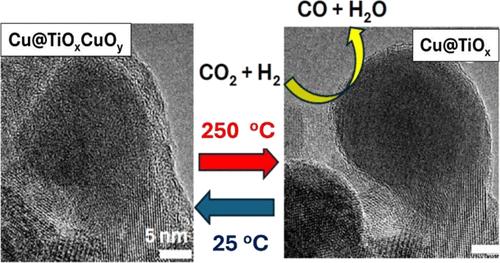
A combination of several in situ techniques (XRD, XAS, AP-XPS, and E-TEM) was used to explore links between the structural and chemical properties of a Cu@TiOx catalyst under CO2 hydrogenation conditions. The active phase of the catalyst involved an inverse oxide/metal configuration, but the initial core@shell motif was disrupted during the pretreatment in H2. As a consequence of strong metal–support interactions, the titania shell cracked, and Cu particles migrated from the core to on top of the oxide with the simultaneous formation of a Cu–Ti–Ox phase. The generated Cu particles had a diameter of 20–40 nm and were decorated by small clusters of TiOx (<5 nm in size). Results of in situ XAS and XRD and images of E-TEM showed a very dynamic system, where the inverse oxide/metal configuration promoted the reactivity of the system toward CO2 and H2. At room temperature, CO2 oxidized the Cu nanoparticles (CO2,gas → COgas + Ooxide) inducing a redistribution of the TiOx clusters and big modifications in catalyst surface morphology. The generated oxide overlayer disappeared at elevated temperatures (>180 °C) upon exposure to H2, producing a transient surface that was very active for the reverse water–gas shift reaction (CO2 + H2 → CO + H2O) but was not stable at 200–350 °C. When oxidation and reduction occurred at the same time, under a mixture of CO2 and H2, the surface structure evolved toward a dynamic equilibrium that strongly depended on the temperature. Neither CO2 nor H2 can be considered as passive reactants. In the Cu@TiOx system, morphological changes were linked to variations in the composition of metal-oxide interfaces which were reversible with temperature or chemical environment and affected the catalytic activity of the system. The present study illustrates the dynamic nature of phenomena associated with the trapping and conversion of CO2.
中文翻译:

观察 Cu@TiOx 核@壳催化剂的化学和形态变化:可逆金属-氧化物相互作用对 CO2 活化和加氢的影响
结合多种原位技术(XRD、XAS、AP-XPS 和 E-TEM)来探索 CO2 2 催化剂的结构和化学性质之间的联系氢化条件。催化剂的活性相涉及相反的氧化物/金属构型,但最初的核@壳基序在H 2 的预处理过程中被破坏。由于金属与载体之间的强烈相互作用,二氧化钛壳破裂,Cu 颗粒从核心迁移到氧化物顶部,同时形成 Cu-Ti-O x 相。生成的 Cu 颗粒直径为 20-40 nm,并由小簇 TiO x (尺寸 <5 nm)装饰。原位 XAS 和 XRD 结果以及 E-TEM 图像显示出一个非常动态的系统,其中反向氧化物/金属构型促进了系统对 CO 2 和 H 2 的反应活性。在室温下,CO 2 氧化 Cu 纳米颗粒(CO 2,gas → CO gas + O oxide ),从而引起 TiO 的重新分布 x 簇和催化剂表面形态的大改变。暴露于 H 2, 后,生成的氧化物覆盖层在高温 (>180 °C) 下消失,产生对于逆水煤气变换反应非常活跃的瞬态表面 (CO 2 + H 2 → CO + H 2 O),但在 200–350 °C 时不稳定。当氧化和还原同时发生时,在 CO 2 和 H 2 的混合物下,表面结构朝着强烈依赖于温度的动态平衡发展。 CO 2 和 H 2 都不能被视为被动反应物。 在Cu@TiO x 体系中,形态变化与金属氧化物界面组成的变化有关,这些变化随温度或化学环境而可逆,并影响系统的催化活性。本研究说明了与 CO 2 捕获和转化相关的现象的动态性质。
更新日期:2024-07-25
中文翻译:

观察 Cu@TiOx 核@壳催化剂的化学和形态变化:可逆金属-氧化物相互作用对 CO2 活化和加氢的影响
结合多种原位技术(XRD、XAS、AP-XPS 和 E-TEM)来探索 CO2 2 催化剂的结构和化学性质之间的联系氢化条件。催化剂的活性相涉及相反的氧化物/金属构型,但最初的核@壳基序在H 2 的预处理过程中被破坏。由于金属与载体之间的强烈相互作用,二氧化钛壳破裂,Cu 颗粒从核心迁移到氧化物顶部,同时形成 Cu-Ti-O x 相。生成的 Cu 颗粒直径为 20-40 nm,并由小簇 TiO x (尺寸 <5 nm)装饰。原位 XAS 和 XRD 结果以及 E-TEM 图像显示出一个非常动态的系统,其中反向氧化物/金属构型促进了系统对 CO 2 和 H 2 的反应活性。在室温下,CO 2 氧化 Cu 纳米颗粒(CO 2,gas → CO gas + O oxide ),从而引起 TiO 的重新分布 x 簇和催化剂表面形态的大改变。暴露于 H 2, 后,生成的氧化物覆盖层在高温 (>180 °C) 下消失,产生对于逆水煤气变换反应非常活跃的瞬态表面 (CO 2 + H 2 → CO + H 2 O),但在 200–350 °C 时不稳定。当氧化和还原同时发生时,在 CO 2 和 H 2 的混合物下,表面结构朝着强烈依赖于温度的动态平衡发展。 CO 2 和 H 2 都不能被视为被动反应物。 在Cu@TiO x 体系中,形态变化与金属氧化物界面组成的变化有关,这些变化随温度或化学环境而可逆,并影响系统的催化活性。本研究说明了与 CO 2 捕获和转化相关的现象的动态性质。












































 京公网安备 11010802027423号
京公网安备 11010802027423号