Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge delocalization and global aromaticity in a partially fused 12-porphyrin nanoring
Chem ( IF 19.1 ) Pub Date : 2024-07-25 , DOI: 10.1016/j.chempr.2024.06.034 Sebastian M. Kopp , Henrik Gotfredsen , Janko Hergenhahn , Arnau Rodríguez-Rubio , Jie-Ren Deng , He Zhu , Wojciech Stawski , Harry L. Anderson
Chem ( IF 19.1 ) Pub Date : 2024-07-25 , DOI: 10.1016/j.chempr.2024.06.034 Sebastian M. Kopp , Henrik Gotfredsen , Janko Hergenhahn , Arnau Rodríguez-Rubio , Jie-Ren Deng , He Zhu , Wojciech Stawski , Harry L. Anderson
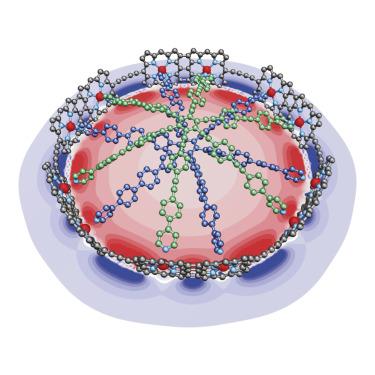
|
Aromatic and antiaromatic ring currents can reveal global electronic delocalization around the circumference of π -conjugated macrocycles, although these phenomena are poorly understood in large rings. Here, we present the template-directed synthesis of a fully π -conjugated cyclic porphyrin 12-mer consisting of six β ,meso ,β -edge-fused porphyrin dimers connected by six butadiyne bridges. The lowest energy π -π ∗ absorption band of this partially fused nanoring is shifted far into the NIR, confirming strong π -conjugation around the circumference of the macrocycle. Investigation of the oxidized and reduced nanoring-template complex by 1 H and 19 F NMR spectroscopy demonstrates the presence of coherent global (anti)aromatic ring currents, consistent with DFT calculations. The stronger π -conjugation enables global charge delocalization even at low levels of oxidation or reduction. These findings open new avenues for the engineering of cyclic molecular wires.
中文翻译:

部分熔合的 12-卟啉纳米环中的电荷离域和全局芳香性
芳香族和反芳香族环流可以揭示π共轭大环周围的整体电子离域,尽管这些现象在大环中知之甚少。在这里,我们提出了完全π偶联的环卟啉 12-mer 的模板定向合成,该二聚体由六个丁二炔桥连接的 6 个 β,中旋,β 边熔合卟啉二聚体组成。这个部分熔融纳米环的最低能量 π-π∗ 吸收带向 NIR 中移动得很远,证实了大环圆周周围的强 π 共轭。通过 1H 和 19F NMR 波谱对氧化和还原的纳米环-模板复合物的研究表明存在相干的全局(反)芳香环电流,这与 DFT 计算一致。更强的 π 共轭即使在低水平的氧化或还原下也能实现全局电荷离域。这些发现为环状分子线的工程设计开辟了新的途径。
更新日期:2024-07-25
中文翻译:

部分熔合的 12-卟啉纳米环中的电荷离域和全局芳香性
芳香族和反芳香族环流可以揭示π共轭大环周围的整体电子离域,尽管这些现象在大环中知之甚少。在这里,我们提出了完全π偶联的环卟啉 12-mer 的模板定向合成,该二聚体由六个丁二炔桥连接的 6 个 β,中旋,β 边熔合卟啉二聚体组成。这个部分熔融纳米环的最低能量 π-π∗ 吸收带向 NIR 中移动得很远,证实了大环圆周周围的强 π 共轭。通过 1H 和 19F NMR 波谱对氧化和还原的纳米环-模板复合物的研究表明存在相干的全局(反)芳香环电流,这与 DFT 计算一致。更强的 π 共轭即使在低水平的氧化或还原下也能实现全局电荷离域。这些发现为环状分子线的工程设计开辟了新的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号