当前位置:
X-MOL 学术
›
Aliment. Pharm. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
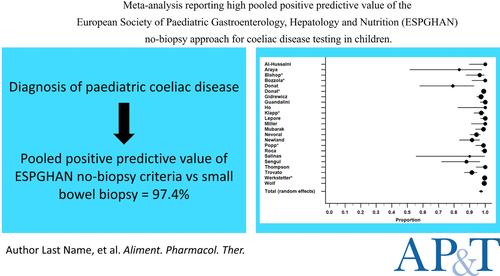
Meta‐analysis: High pooled positive predictive value of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition no‐biopsy approach for coeliac disease testing in children
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-07-24 , DOI: 10.1111/apt.18177 Angharad Vernon‐Roberts 1 , Sanjeev Verma 2 , Andrew S. Day 1 , Shaun S. C. Ho 3, 4, 5
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-07-24 , DOI: 10.1111/apt.18177 Angharad Vernon‐Roberts 1 , Sanjeev Verma 2 , Andrew S. Day 1 , Shaun S. C. Ho 3, 4, 5
Affiliation
|
SummaryBackgroundThe European Society of Paediatric Gastroenterology, Hepatology and Nutrition established guidelines in 2012 for a no‐biopsy approach to diagnose coeliac disease in children. This guideline required symptoms suggestive of coeliac disease, positive human leukocyte antigen (HLA) DQ2/DQ8 haplotypes, tissue transglutaminase type‐2 immunoglobulin A antibody titre at levels greater than 10 times the upper limit of normal, and positive endomysial immune‐globulin A antibody test. An updated 2020 guideline excluded the need for symptoms and positive HLA.AimsTo assess the pooled positive predictive value (PPV) of the no‐biopsy approach with small bowel biopsy (SBB) data as the reference standard for comparison.MethodsDatabase searches (October 2023) provided data that we combined using a random‐effects meta‐analysis to provide a pooled PPV, representing the probability that a positive test result means that an individual truly has the condition.ResultsWe included 23 studies. Study sample sizes totalled 23,769 but only 3007 children had comparative SBB. The proportion of coeliac disease confirmed by the no‐biopsy approach and SBB ranged from 79.2% to 100%, with an overall pooled PPV of 97.4% (95% confidence interval 96.0, 98.6). Sensitivity analysis showed higher PPV for the criteria that included HLA (98.5% vs. 96.8%; p = 0.017).ConclusionBoth no‐biopsy criteria exhibit high PPV when compared to the reference standard. These results provide a consistent message of accuracy and feasibility to inform change and improve outcomes.
中文翻译:

荟萃分析:欧洲儿科胃肠病学、肝病学和营养学会用于儿童乳糜泻检测的非活检方法的高汇总阳性预测价值
摘要背景欧洲儿科胃肠病学、肝病学和营养学会于 2012 年制定了用于诊断儿童乳糜泻的非活检方法的指南。该指南要求出现提示乳糜泻的症状、人类白细胞抗原 (HLA) DQ2/DQ8 单倍型阳性、组织转谷氨酰胺酶 2 型免疫球蛋白 A 抗体滴度水平大于正常上限 10 倍、肌内膜免疫球蛋白 A 抗体阳性测试。 2020 年更新的指南排除了症状和 HLA 阳性的必要性。目的以小肠活检 (SBB) 数据作为比较的参考标准,评估非活检方法的汇总阳性预测值 (PPV)。方法数据库搜索(2023 年 10 月)我们使用随机效应荟萃分析结合提供的数据来提供汇总 PPV,代表阳性测试结果意味着个体确实患有该病症的概率。结果我们纳入了 23 项研究。研究样本总数为 23,769 人,但只有 3007 名儿童具有相对 SBB。通过非活检方法和 SBB 确诊的乳糜泻比例为 79.2% 至 100%,总体合并 PPV 为 97.4%(95% 置信区间 96.0, 98.6)。敏感性分析显示,包含 HLA 的标准的 PPV 较高(98.5% vs. 96.8%; p = 0.017)。结论与参考标准相比,两种非活检标准均表现出较高的 PPV。这些结果提供了一致的准确性和可行性信息,以告知变革和改善结果。
更新日期:2024-07-24
中文翻译:

荟萃分析:欧洲儿科胃肠病学、肝病学和营养学会用于儿童乳糜泻检测的非活检方法的高汇总阳性预测价值
摘要背景欧洲儿科胃肠病学、肝病学和营养学会于 2012 年制定了用于诊断儿童乳糜泻的非活检方法的指南。该指南要求出现提示乳糜泻的症状、人类白细胞抗原 (HLA) DQ2/DQ8 单倍型阳性、组织转谷氨酰胺酶 2 型免疫球蛋白 A 抗体滴度水平大于正常上限 10 倍、肌内膜免疫球蛋白 A 抗体阳性测试。 2020 年更新的指南排除了症状和 HLA 阳性的必要性。目的以小肠活检 (SBB) 数据作为比较的参考标准,评估非活检方法的汇总阳性预测值 (PPV)。方法数据库搜索(2023 年 10 月)我们使用随机效应荟萃分析结合提供的数据来提供汇总 PPV,代表阳性测试结果意味着个体确实患有该病症的概率。结果我们纳入了 23 项研究。研究样本总数为 23,769 人,但只有 3007 名儿童具有相对 SBB。通过非活检方法和 SBB 确诊的乳糜泻比例为 79.2% 至 100%,总体合并 PPV 为 97.4%(95% 置信区间 96.0, 98.6)。敏感性分析显示,包含 HLA 的标准的 PPV 较高(98.5% vs. 96.8%; p = 0.017)。结论与参考标准相比,两种非活检标准均表现出较高的 PPV。这些结果提供了一致的准确性和可行性信息,以告知变革和改善结果。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号