Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and characterization of tetra substituted 2,3-dihydrothiazole derivatives as DNA and BSA targeting agents: advantages of the visible-light-induced multicomponent approach
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra02331e Ranjana Aggarwal 1, 2 , Naman Jain 1 , Gyan Prakash Dubey 1
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra02331e Ranjana Aggarwal 1, 2 , Naman Jain 1 , Gyan Prakash Dubey 1
Affiliation
|
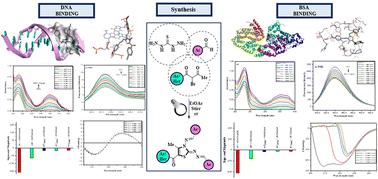
This report describes the visible-light-induced one-pot multicomponent regioselective synthesis of a series of 5-aroyl-3-((arylidene)amino)-2-((arylidene)hydrazono)-4-methyl-2,3-dihydrothiazoles as DNA and BSA targeting agents. The multicomponent condensation of thiocarbohydrazide and aldehydes with α-bromo-1,3-diketones, generated in situ by the bromination of unsymmetrical 1,3-diketones with NBS using white LED light as an environmental friendly source in the presence of EtOAc solvent furnished the titled 2,3-dihydrothiazole derivatives in excellent yields. The exact regioisomeric structure was identified unambiguously by employing multinuclear 2D-NMR spectroscopy [1H–13C] HMBC; [1H–13C] HMQC and [1H–15N] HMBC. Furthermore, the binding characteristics of the synthesized 2,3-dihydrothiazole derivatives were assessed with double-stranded calf-thymus DNA duplex (ct-DNA) and bovine serum albumin (BSA). Initial screening of all the synthesized 2,3-dihydrothiazole derivatives using various in silico techniques including molecular reactivity analysis, Lipinski rule and molecular docking, concluded 5-(4′-chlorobenzoyl)-3-((4′′-methoxybenzylidene)amino)-2-(4′′′-methoxybenzylidene)hydrazono)-4-methyl-2,3-dihydrothiazole derivative 6a as the most suitable compound for studying binding interaction with DNA and BSA. Additionally, to illustrate the ex vivo binding mode of 6a with DNA and BSA, several spectroscopic techniques viz. UV-visible, circular dichroism (CD), steady-state fluorescence and competitive displacement assays were carried out.
中文翻译:

作为 DNA 和 BSA 靶向剂的四取代 2,3-二氢噻唑衍生物的设计、合成和表征:可见光诱导多组分方法的优点
该报告描述了一系列5-芳酰基-3-((亚芳基)氨基)-2-((亚芳基)亚肼基)-4-甲基-2,3-二氢噻唑的可见光诱导一锅多组分区域选择性合成作为 DNA 和 BSA 靶向剂。硫代碳酰肼和醛与 α-溴-1,3-二酮的多组分缩合,通过不对称 1,3-二酮与 NBS 的溴化原位生成,使用白色 LED 光作为环保光源,在 EtOAc 溶剂存在下,提供了标题为 2,3-二氢噻唑衍生物,收率优异。通过使用多核 2D-NMR 光谱 [ 1 H– 13 C] HMBC 明确鉴定了确切的区域异构结构; [ 1 H– 13 C] HMQC 和 [ 1 H– 15 N] HMBC。此外,使用双链小牛胸腺 DNA 双链体 (ct-DNA) 和牛血清白蛋白 (BSA) 评估了合成的 2,3-二氢噻唑衍生物的结合特性。使用分子反应性分析、Lipinski规则和分子对接等各种计算机技术对所有合成的2,3-二氢噻唑衍生物进行初步筛选,得出5-(4'-氯苯甲酰基)-3-((4''-甲氧基亚苄基)氨基) -2-(4'''-甲氧基亚苯亚甲基)亚肼基)-4-甲基-2,3-二氢噻唑衍生物6a是最适合研究DNA和BSA结合相互作用的化合物。此外,为了说明6a与 DNA 和 BSA 的离体结合模式,采用了几种光谱技术,即。进行了紫外可见光、圆二色性(CD)、稳态荧光和竞争性位移测定。
更新日期:2024-07-23
中文翻译:

作为 DNA 和 BSA 靶向剂的四取代 2,3-二氢噻唑衍生物的设计、合成和表征:可见光诱导多组分方法的优点
该报告描述了一系列5-芳酰基-3-((亚芳基)氨基)-2-((亚芳基)亚肼基)-4-甲基-2,3-二氢噻唑的可见光诱导一锅多组分区域选择性合成作为 DNA 和 BSA 靶向剂。硫代碳酰肼和醛与 α-溴-1,3-二酮的多组分缩合,通过不对称 1,3-二酮与 NBS 的溴化原位生成,使用白色 LED 光作为环保光源,在 EtOAc 溶剂存在下,提供了标题为 2,3-二氢噻唑衍生物,收率优异。通过使用多核 2D-NMR 光谱 [ 1 H– 13 C] HMBC 明确鉴定了确切的区域异构结构; [ 1 H– 13 C] HMQC 和 [ 1 H– 15 N] HMBC。此外,使用双链小牛胸腺 DNA 双链体 (ct-DNA) 和牛血清白蛋白 (BSA) 评估了合成的 2,3-二氢噻唑衍生物的结合特性。使用分子反应性分析、Lipinski规则和分子对接等各种计算机技术对所有合成的2,3-二氢噻唑衍生物进行初步筛选,得出5-(4'-氯苯甲酰基)-3-((4''-甲氧基亚苄基)氨基) -2-(4'''-甲氧基亚苯亚甲基)亚肼基)-4-甲基-2,3-二氢噻唑衍生物6a是最适合研究DNA和BSA结合相互作用的化合物。此外,为了说明6a与 DNA 和 BSA 的离体结合模式,采用了几种光谱技术,即。进行了紫外可见光、圆二色性(CD)、稳态荧光和竞争性位移测定。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号