当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reductive activation of arenes by potassium metal with potassium salts
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-23 , DOI: 10.1039/d4qo01027b Giuseppe Nocera , Iain Robb , Kenneth F. Clark , Thomas McGuire , Laura Evans , Shunsuke Chiba , John Murphy
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-23 , DOI: 10.1039/d4qo01027b Giuseppe Nocera , Iain Robb , Kenneth F. Clark , Thomas McGuire , Laura Evans , Shunsuke Chiba , John Murphy
|
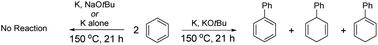
Benzene is routinely dried by refluxing over potassium or other alkali metals and is inert to the metal. However, dramatic chemistry occurs when potassium salts are added. At 150 °C, dimerisation occurs to afford biphenyl as the product. In the absence of salt, no reaction occurs. We propose that the process is initiated by activation of the arene by the salt followed by electron transfer from potassium. In support of this, within the added salt, systematic alteration of (i) the anion and (ii) the cation shows that the cation is the important component; thus, K+ is effective but Na+ and Li+ are not. Studies with a mixture of benzene and benzene-d6 show facile transfer of H−/D− ions between molecules during the reaction. Extension of the study to other arene hydrocarbons shows the generality of the chemistry.
中文翻译:

钾金属与钾盐对芳烃的还原活化
苯通常通过在钾或其他碱金属上回流来干燥,并且对金属呈惰性。然而,当添加钾盐时,会发生戏剧性的化学反应。在 150 °C 时,发生二聚,得到联苯产物。在没有盐的情况下,不会发生反应。我们建议该过程是通过盐活化芳烃,然后从钾转移电子来引发的。为了支持这一点,在添加的盐中,(i)阴离子和(ii)阳离子的系统改变表明阳离子是重要的成分;因此,K + 有效,但 Na + 和 Li + 无效。对苯和苯-d 6 混合物的研究表明,反应过程中 H − /D − 离子在分子之间很容易转移。将研究扩展到其他芳烃烃显示了化学的普遍性。
更新日期:2024-07-26
中文翻译:

钾金属与钾盐对芳烃的还原活化
苯通常通过在钾或其他碱金属上回流来干燥,并且对金属呈惰性。然而,当添加钾盐时,会发生戏剧性的化学反应。在 150 °C 时,发生二聚,得到联苯产物。在没有盐的情况下,不会发生反应。我们建议该过程是通过盐活化芳烃,然后从钾转移电子来引发的。为了支持这一点,在添加的盐中,(i)阴离子和(ii)阳离子的系统改变表明阳离子是重要的成分;因此,K + 有效,但 Na + 和 Li + 无效。对苯和苯-d 6 混合物的研究表明,反应过程中 H − /D − 离子在分子之间很容易转移。将研究扩展到其他芳烃烃显示了化学的普遍性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号