当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Electrocatalysis on Copper Nanostructures: Role of the Oxidation State in Sulfite Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-19 , DOI: 10.1021/acscatal.3c05897 Esperanza Fernández-García 1 , Pablo Merino 2 , Nerea González-Rodríguez 1 , Lidia Martínez 2 , María Del Pozo 1 , Javier Prieto 2 , Elías Blanco 1 , Gonzalo Santoro 3 , Carmen Quintana 1 , María Dolores Petit-Domínguez 1 , Elena Casero 1 , Luis Vázquez 2 , José I Martínez 2 , José A Martín-Gago 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-19 , DOI: 10.1021/acscatal.3c05897 Esperanza Fernández-García 1 , Pablo Merino 2 , Nerea González-Rodríguez 1 , Lidia Martínez 2 , María Del Pozo 1 , Javier Prieto 2 , Elías Blanco 1 , Gonzalo Santoro 3 , Carmen Quintana 1 , María Dolores Petit-Domínguez 1 , Elena Casero 1 , Luis Vázquez 2 , José I Martínez 2 , José A Martín-Gago 2
Affiliation
|
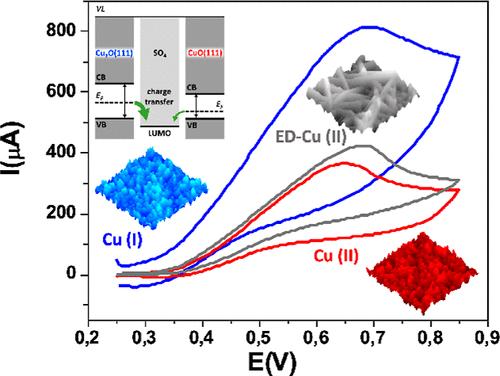
The influence of surface morphology and the oxidation state on the electrocatalytic activity of nanostructured electrodes is well recognized, yet disentangling their individual roles in specific reactions remains challenging. Here, we investigated the electrooxidation of sulfite ions in an alkaline environment using cyclic voltammetry on copper oxide nanostructured electrodes with different oxidation states and morphologies but with similar active areas. To this aim, we synthesized nanostructured Cu films made of nanoparticles or nanorods on top of glassy carbon electrodes. Our findings showed an enhanced sensitivity and a lower detection threshold when utilizing Cu(I) over Cu(II). Density functional theory-based thermochemical analysis revealed the underlying oxidation mechanism, indicating that while the energy gain associated with the process is comparable for both oxide surfaces, the desorption energy barrier for the resulting sulfate molecules is three times higher on Cu(II). This becomes the limiting step of the reaction kinetics and diminishes the overall electrooxidation efficiency. Our proposed mechanism relies on the tautomerization of hydroxyl groups confined on the surface of Cu-based electrodes. This mechanism might be applicable to electrochemical reactions involving other sulfur compounds that hold technological significance.
中文翻译:

铜纳米结构的增强电催化:氧化态在亚硫酸盐氧化中的作用
表面形态和氧化态对纳米结构电极电催化活性的影响已得到广泛认可,但阐明它们在特定反应中的各自作用仍然具有挑战性。在这里,我们使用循环伏安法在具有不同氧化态和形貌但具有相似活性区域的氧化铜纳米结构电极上研究了亚硫酸根离子在碱性环境中的电氧化。为此,我们在玻碳电极顶部合成了由纳米颗粒或纳米棒制成的纳米结构铜膜。我们的研究结果表明,使用 Cu(I) 比使用 Cu(II) 时的灵敏度更高,检测阈值更低。基于密度泛函理论的热化学分析揭示了潜在的氧化机制,表明虽然两个氧化物表面与该过程相关的能量增益相当,但所得硫酸盐分子的解吸能垒是 Cu(II) 上的三倍。这成为反应动力学的限制步骤并降低了整体电氧化效率。我们提出的机制依赖于限制在铜基电极表面的羟基的互变异构。该机制可能适用于涉及其他具有技术意义的硫化合物的电化学反应。
更新日期:2024-07-19
中文翻译:

铜纳米结构的增强电催化:氧化态在亚硫酸盐氧化中的作用
表面形态和氧化态对纳米结构电极电催化活性的影响已得到广泛认可,但阐明它们在特定反应中的各自作用仍然具有挑战性。在这里,我们使用循环伏安法在具有不同氧化态和形貌但具有相似活性区域的氧化铜纳米结构电极上研究了亚硫酸根离子在碱性环境中的电氧化。为此,我们在玻碳电极顶部合成了由纳米颗粒或纳米棒制成的纳米结构铜膜。我们的研究结果表明,使用 Cu(I) 比使用 Cu(II) 时的灵敏度更高,检测阈值更低。基于密度泛函理论的热化学分析揭示了潜在的氧化机制,表明虽然两个氧化物表面与该过程相关的能量增益相当,但所得硫酸盐分子的解吸能垒是 Cu(II) 上的三倍。这成为反应动力学的限制步骤并降低了整体电氧化效率。我们提出的机制依赖于限制在铜基电极表面的羟基的互变异构。该机制可能适用于涉及其他具有技术意义的硫化合物的电化学反应。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号