当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Distance-Dependent Charge Redistribution Boosts Hydrogen Evolution in Hybrid Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-19 , DOI: 10.1021/acscatal.4c01396 Xinzhang Lin 1, 2 , Yifan Li 3 , Wei Tu 4 , Zhenyu Li 2 , Xiang Li 2 , Dongze Li 1 , Nanfeng Xu 1 , Chao Wang 1 , Yi Lu 1 , Song Jin 5 , Hengxing Ji 5 , Wei Liu 4 , Guoxiong Wang 2 , Junyuan Xu 1 , Zhangquan Peng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-19 , DOI: 10.1021/acscatal.4c01396 Xinzhang Lin 1, 2 , Yifan Li 3 , Wei Tu 4 , Zhenyu Li 2 , Xiang Li 2 , Dongze Li 1 , Nanfeng Xu 1 , Chao Wang 1 , Yi Lu 1 , Song Jin 5 , Hengxing Ji 5 , Wei Liu 4 , Guoxiong Wang 2 , Junyuan Xu 1 , Zhangquan Peng 1
Affiliation
|
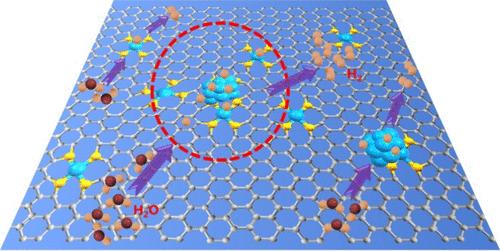
The hybrid catalysts have demonstrated decent catalytic performance for hydrogen evolution reaction (HER), but the specific roles and interactions of different species contributing to the activity remain ambiguous. Here we synthesized Pt hybrid catalysts through the ligand preprotected pyrolysis method, which has demonstrated its universality for preparing Ir and Ru hybrid catalysts due to the unique constraining and pulling effects exerted by ligands. The hybrid catalysts demonstrated higher activity than single-component catalysts, such as Pt single atoms or Pt nanoparticles. Particularly, the Pt/CNT-600 hybrid catalyst displayed good HER activity with a low overpotential of 15 mV at −10 mA cm–2, and its mass activity was 8 times than that of Pt/C-20%. In hybrid catalysts, the synergistic effect via charge redistribution in the Pt1 and Ptn sites afforded the optimal Pt–H binding energy for fast HER kinetics. More importantly, it presented an intense dependence of charge distribution on the distance between Pt1 and Ptn sites, and the volcanic curve indicated the optimal distance of approximately 0.43 nm for the synergistic effect. Consequently, we proposed that the Ptn-mPt1 active units offered the dominant contribution to catalytic activity, resulting from the collective synergistic effect between Ptn and every adjacent Pt1 site. This study not only presents a universal method for synthesizing hybrid catalysts but also offers fresh insights into their rational design and activity enhancement by manipulating the intersite distance and interaction effect.
中文翻译:

距离相关的电荷重新分布促进混合催化剂中的氢析出
混合催化剂在析氢反应(HER)中表现出良好的催化性能,但不同物种对该活性的具体作用和相互作用仍然不明确。在这里,我们通过配体预保护热解法合成了Pt杂化催化剂,由于配体发挥的独特的约束和牵引作用,该方法证明了其在制备Ir和Ru杂化催化剂中的普适性。混合催化剂表现出比单组分催化剂(例如 Pt 单原子或 Pt 纳米颗粒)更高的活性。特别是,Pt/CNT-600杂化催化剂在-10 mA cm –2 下表现出良好的HER活性,过电势仅为15 mV –2 ,其质量活性是Pt/C-20%的8倍。 。在混合催化剂中,通过 Pt 1 和 Pt n 位点的电荷重新分布产生的协同效应为快速 HER 动力学提供了最佳的 Pt-H 结合能。更重要的是,它表现出电荷分布对 Pt 1 和 Pt n 位点之间距离的强烈依赖性,火山曲线表明协同效应的最佳距离约为 0.43 nm 。因此,我们提出,Pt n -mPt 1 活性单元对催化活性提供了主导贡献,这是由于 Pt n 和每个活性单元之间的集体协同效应所致。邻近的 Pt 1 站点。这项研究不仅提出了合成混合催化剂的通用方法,而且还为通过操纵位点距离和相互作用效应来合理设计和增强活性提供了新的见解。
更新日期:2024-07-22
中文翻译:

距离相关的电荷重新分布促进混合催化剂中的氢析出
混合催化剂在析氢反应(HER)中表现出良好的催化性能,但不同物种对该活性的具体作用和相互作用仍然不明确。在这里,我们通过配体预保护热解法合成了Pt杂化催化剂,由于配体发挥的独特的约束和牵引作用,该方法证明了其在制备Ir和Ru杂化催化剂中的普适性。混合催化剂表现出比单组分催化剂(例如 Pt 单原子或 Pt 纳米颗粒)更高的活性。特别是,Pt/CNT-600杂化催化剂在-10 mA cm –2 下表现出良好的HER活性,过电势仅为15 mV –2 ,其质量活性是Pt/C-20%的8倍。 。在混合催化剂中,通过 Pt 1 和 Pt n 位点的电荷重新分布产生的协同效应为快速 HER 动力学提供了最佳的 Pt-H 结合能。更重要的是,它表现出电荷分布对 Pt 1 和 Pt n 位点之间距离的强烈依赖性,火山曲线表明协同效应的最佳距离约为 0.43 nm 。因此,我们提出,Pt n -mPt 1 活性单元对催化活性提供了主导贡献,这是由于 Pt n 和每个活性单元之间的集体协同效应所致。邻近的 Pt 1 站点。这项研究不仅提出了合成混合催化剂的通用方法,而且还为通过操纵位点距离和相互作用效应来合理设计和增强活性提供了新的见解。












































 京公网安备 11010802027423号
京公网安备 11010802027423号