当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Glycosylation via Halogen-Atom-Transfer for C-Glycoside Assembly
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-19 , DOI: 10.1021/acscatal.4c02322 Jun Wu 1 , Rajeshwaran Purushothaman 1 , Felix Kallert 1 , Simon L. Homölle 1 , Lutz Ackermann 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-19 , DOI: 10.1021/acscatal.4c02322 Jun Wu 1 , Rajeshwaran Purushothaman 1 , Felix Kallert 1 , Simon L. Homölle 1 , Lutz Ackermann 1
Affiliation
|
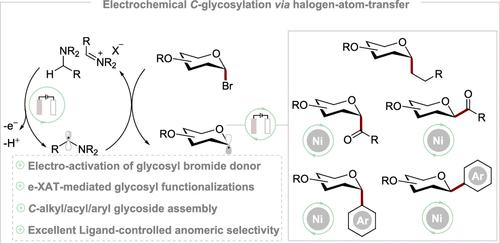
Glycosyl donor activation emerged as an enabling technology for anomeric functionalization, but aimed primarily at O-glycosylation. In contrast, we herein disclose mechanistically distinct electrochemical glycosyl bromide donor activations via halogen-atom transfer and anomeric C-glycosylation. The anomeric radical addition to alkenes led to C-alkyl glycoside synthesis under precious metal-free reaction conditions from readily available glycosyl bromides. The robustness of our e-XAT strategy was further mirrored by C-aryl and C-acyl glycosides assembly through nickela-electrocatalysis. Our approach provides an orthogonal strategy for glycosyl donor activation with expedient scope, hence representing a general method for direct C-glycosides assembly.
中文翻译:

通过卤素原子转移进行电化学糖基化以进行 C-糖苷组装
糖基供体激活是作为异头功能化的一项可行技术而出现的,但主要针对 O-糖基化。相反,我们在此公开了通过卤素原子转移和异头C-糖基化的机械上不同的电化学糖基溴供体激活。异头自由基加成到烯烃上导致在无贵金属的反应条件下从容易获得的糖基溴合成C-烷基糖苷。我们的 e-XAT 策略的稳健性通过镍电催化的 C-芳基和 C-酰基糖苷组装得到了进一步体现。我们的方法为糖基供体激活提供了一种正交策略,具有方便的范围,因此代表了直接 C-糖苷组装的通用方法。
更新日期:2024-07-22
中文翻译:

通过卤素原子转移进行电化学糖基化以进行 C-糖苷组装
糖基供体激活是作为异头功能化的一项可行技术而出现的,但主要针对 O-糖基化。相反,我们在此公开了通过卤素原子转移和异头C-糖基化的机械上不同的电化学糖基溴供体激活。异头自由基加成到烯烃上导致在无贵金属的反应条件下从容易获得的糖基溴合成C-烷基糖苷。我们的 e-XAT 策略的稳健性通过镍电催化的 C-芳基和 C-酰基糖苷组装得到了进一步体现。我们的方法为糖基供体激活提供了一种正交策略,具有方便的范围,因此代表了直接 C-糖苷组装的通用方法。












































 京公网安备 11010802027423号
京公网安备 11010802027423号