当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Role of Particle Size and Nanostructuring on the Oxygen Evolution Activity of Fe-Doped NiO
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-16 , DOI: 10.1021/acscatal.4c02329 Reshma R. Rao 1, 2 , Alberto Bucci 3 , Sacha Corby 4 , Benjamin Moss 4 , Caiwu Liang 1 , Aswin Gopakumar 3 , Ifan E. L. Stephens 1 , Julio Lloret-Fillol 3, 5 , James R. Durrant 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-16 , DOI: 10.1021/acscatal.4c02329 Reshma R. Rao 1, 2 , Alberto Bucci 3 , Sacha Corby 4 , Benjamin Moss 4 , Caiwu Liang 1 , Aswin Gopakumar 3 , Ifan E. L. Stephens 1 , Julio Lloret-Fillol 3, 5 , James R. Durrant 4
Affiliation
|
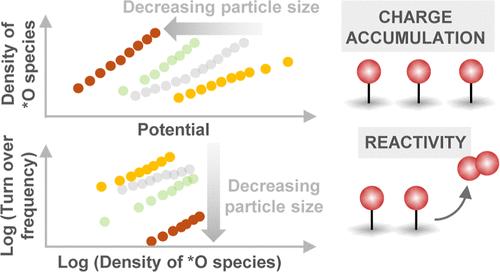
Nickel-based oxides and oxyhydroxide catalysts exhibit state-of-the-art activity for the sluggish oxygen evolution reaction (OER) under alkaline conditions. A widely employed strategy to increase the gravimetric activity of the catalyst is to increase the active surface area via nanostructuring or decrease the particle size. However, the fundamental understanding about how tuning these parameters influences the density of oxidized species and their reaction kinetics remains unclear. Here, we use solution combustion synthesis, a low-cost and scalable approach, to synthesize a series of Fe0.1Ni0.9O samples from different precursor salts. Based on the precursor salt, the nanoparticle size can be changed significantly from ∼2.5 to ∼37 nm. The OER activity at pH 13 trends inversely with the particle size. Using operando time-resolved optical spectroscopy, we quantify the density of oxidized species as a function of potential and demonstrate that the OER kinetics exhibits a second-order dependence on the density of these species, suggesting that the OER mechanism relies on O–O coupling between neighboring oxidized species. With the decreasing particle size, the density of species accumulated is found to increase, and their intrinsic reactivity for the OER is found to decrease, attributed to the stronger binding of *O species (i.e., a cathodic shift of species energetics). This signifies that the high apparent OER activity per geometric area of the smaller nanoparticles is driven by their ability to accumulate a larger density of oxidized species. This study not only experimentally disentangles the influence of the density of oxidized species and intrinsic kinetics on the overall rate of the OER but also highlights the importance of tuning these parameters independently to develop more active OER catalysts.
中文翻译:

揭示粒径和纳米结构对 Fe 掺杂 NiO 析氧活性的作用
镍基氧化物和羟基氧化物催化剂在碱性条件下缓慢的析氧反应 (OER) 中表现出最先进的活性。提高催化剂重量活性的一种广泛采用的策略是通过纳米结构增加活性表面积或减小颗粒尺寸。然而,关于调整这些参数如何影响氧化物质的密度及其反应动力学的基本理解仍不清楚。在这里,我们使用溶液燃烧合成这种低成本且可扩展的方法,从不同的前体盐合成一系列 Fe 0.1 Ni 0.9 O 样品。基于前体盐,纳米颗粒尺寸可以从~2.5 nm显着改变到~37 nm。 pH 13 时的 OER 活性与粒径呈反比。使用操作时间分辨光谱,我们量化了氧化物质的密度作为电势的函数,并证明 OER 动力学表现出对这些物质密度的二阶依赖性,表明 OER 机制依赖于 O-O 耦合相邻氧化物种之间。随着粒径的减小,积累的物质密度增加,并且它们对 OER 的内在反应性降低,这归因于 *O 物质的结合更强(即物质能量的阴极转变)。这意味着较小纳米颗粒单位几何面积的高表观 OER 活性是由它们积累较大密度氧化物质的能力驱动的。 这项研究不仅通过实验阐明了氧化物质密度和内在动力学对 OER 总体速率的影响,而且强调了独立调整这些参数以开发更具活性的 OER 催化剂的重要性。
更新日期:2024-07-16
中文翻译:

揭示粒径和纳米结构对 Fe 掺杂 NiO 析氧活性的作用
镍基氧化物和羟基氧化物催化剂在碱性条件下缓慢的析氧反应 (OER) 中表现出最先进的活性。提高催化剂重量活性的一种广泛采用的策略是通过纳米结构增加活性表面积或减小颗粒尺寸。然而,关于调整这些参数如何影响氧化物质的密度及其反应动力学的基本理解仍不清楚。在这里,我们使用溶液燃烧合成这种低成本且可扩展的方法,从不同的前体盐合成一系列 Fe 0.1 Ni 0.9 O 样品。基于前体盐,纳米颗粒尺寸可以从~2.5 nm显着改变到~37 nm。 pH 13 时的 OER 活性与粒径呈反比。使用操作时间分辨光谱,我们量化了氧化物质的密度作为电势的函数,并证明 OER 动力学表现出对这些物质密度的二阶依赖性,表明 OER 机制依赖于 O-O 耦合相邻氧化物种之间。随着粒径的减小,积累的物质密度增加,并且它们对 OER 的内在反应性降低,这归因于 *O 物质的结合更强(即物质能量的阴极转变)。这意味着较小纳米颗粒单位几何面积的高表观 OER 活性是由它们积累较大密度氧化物质的能力驱动的。 这项研究不仅通过实验阐明了氧化物质密度和内在动力学对 OER 总体速率的影响,而且强调了独立调整这些参数以开发更具活性的 OER 催化剂的重要性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号