当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Spiro[cyclopentane-1,3′-oxindole] Scaffolds with Five Consecutive Stereocenters
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.orglett.4c02173
Qiliang Luo 1 , Tao Mao 1 , Yao Luo 1 , Yuxin Zhang 1 , Fei Wang 1 , Shunxi Dong 1 , Xiaoming Feng 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.orglett.4c02173
Qiliang Luo 1 , Tao Mao 1 , Yao Luo 1 , Yuxin Zhang 1 , Fei Wang 1 , Shunxi Dong 1 , Xiaoming Feng 1
Affiliation
|
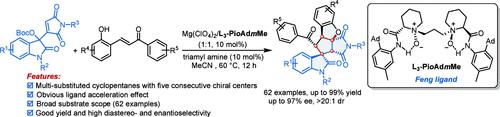
A highly diastereo- and enantioselective cascade annulation reaction of Morita–Baylis–Hillman (MBH) maleimides of isatins with ortho-hydroxychalcones was achieved by a chiral N,N′-dioxide/Mg(II) complex Lewis acid catalyst. This strategy provides a concise and efficient route to densely functionalized spiro[cyclopentane-1,3′-oxindole] compounds with five consecutive stereocenters. The reaction itself features mild conditions, good functional group compatibility, and broad substrate scope (62 examples, up to 99% yield, up to >20:1 dr, 97% ee). In addition, an obvious ligand acceleration effect and chiral amplification effect were observed. DFT calculations were performed to elucidate the stereoselectivity observed. The gram-scale synthesis and the inhibitory effect of two products on the viability of A549 cells demonstrate the potential utility of the current method.
中文翻译:

具有五个连续立构中心的螺[环戊烷-1,3′-羟吲哚]支架的对映选择性合成
通过手性N,N'-二氧化物/Mg(II)络合物路易斯酸催化剂,实现了靛红的 Morita-Baylis-Hillman (MBH) 马来酰亚胺与邻羟基查耳酮的高度非对映和对映选择性级联成环反应。该策略为具有五个连续立体中心的密集官能化螺[环戊烷-1,3'-羟吲哚]化合物提供了简洁有效的途径。该反应本身具有条件温和、官能团相容性好、底物范围广(62个实例,收率高达99%,高达>20:1 dr,97% ee)的特点。此外,还观察到明显的配体加速效应和手性放大效应。进行 DFT 计算以阐明观察到的立体选择性。两种产品的克级合成和对 A549 细胞活力的抑制作用证明了当前方法的潜在效用。
更新日期:2024-07-22
中文翻译:

具有五个连续立构中心的螺[环戊烷-1,3′-羟吲哚]支架的对映选择性合成
通过手性N,N'-二氧化物/Mg(II)络合物路易斯酸催化剂,实现了靛红的 Morita-Baylis-Hillman (MBH) 马来酰亚胺与邻羟基查耳酮的高度非对映和对映选择性级联成环反应。该策略为具有五个连续立体中心的密集官能化螺[环戊烷-1,3'-羟吲哚]化合物提供了简洁有效的途径。该反应本身具有条件温和、官能团相容性好、底物范围广(62个实例,收率高达99%,高达>20:1 dr,97% ee)的特点。此外,还观察到明显的配体加速效应和手性放大效应。进行 DFT 计算以阐明观察到的立体选择性。两种产品的克级合成和对 A549 细胞活力的抑制作用证明了当前方法的潜在效用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号