当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electroreduction of Captured CO2 on Silver Catalysts: Influence of the Capture Agent and Proton Source
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-22 , DOI: 10.1021/jacs.4c03915 Robert Michael Kowalski 1 , Avishek Banerjee 1 , Chudi Yue 1 , Sara G. Gracia 1 , Dongfang Cheng 1 , Carlos G. Morales-Guio 1 , Philippe Sautet 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-22 , DOI: 10.1021/jacs.4c03915 Robert Michael Kowalski 1 , Avishek Banerjee 1 , Chudi Yue 1 , Sara G. Gracia 1 , Dongfang Cheng 1 , Carlos G. Morales-Guio 1 , Philippe Sautet 1, 2, 3
Affiliation
|
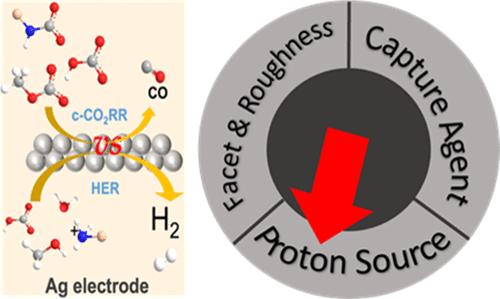
In the context of carbon reutilization, the direct electroreduction of captured CO2 (c-CO2RR) appears as an appealing approach since it avoids the energetically costly separation of CO2 from the capture agent. In this process, CO2 is directly reduced from its captured form. Here, we investigate the influence of the capture agent and proton source on that reaction from a combination of theory and experiment. Specifically, we consider methoxide-captured CO2, NH3-captured CO2, and bicarbonate on silver electrocatalysts. We show that the proton source plays a key role in the interplay of the chemistries for the electroreduction of protons, free CO2, and captured CO2. Our density functional theory calculations, including the influence of the potential, demonstrate that a proton source with smaller pKa improves the reactivity for c-CO2RR, but also increases the selectivity toward the hydrogen evolution reaction (HER) on silver surfaces. Since c-CO2RR requires an additional chemical protonation step, the influence of the proton source is stronger than that of the HER. However, c-CO2RR cannot compete with the HER on Ag, Experimentally, the dominant product observed is H2 with low amounts of CO being produced. Through a rotating cylinder electrode cell of well-defined mass-transport properties, we conclude that although methanol solvent exhibits a lower HER activity, HER remains dominant over c-CO2RR. Our work suggests that methoxide is a potential alternative capture agent to NH3 for direct reduction of captured CO2, though challenges in catalyst design, particularly in reducing the onset potential of c-CO2RR to surpass the HER, remain to be addressed.
中文翻译:

捕获的 CO2 在银催化剂上的电还原:捕获剂和质子源的影响
在碳再利用的背景下,捕获的 CO 2 (c-CO 2 RR) 的直接电还原似乎是一种有吸引力的方法,因为它避免了能源成本高昂的 CO 2 来自捕获代理。在此过程中,CO 2 从其捕获形式直接还原。在这里,我们结合理论和实验研究了捕获剂和质子源对该反应的影响。具体来说,我们考虑银电催化剂上的甲醇盐捕获的 CO 2 、NH 3 捕获的 CO 2 和碳酸氢盐。我们表明,质子源在质子电还原、游离 CO 2 和捕获的 CO 2 化学反应的相互作用中起着关键作用。我们的密度泛函理论计算(包括电势的影响)表明,具有较小 pK a 的质子源提高了 c-CO 2 RR 的反应性,同时也提高了对银表面的析氢反应(HER)。由于c-CO 2 RR需要额外的化学质子化步骤,因此质子源的影响比HER的影响更强。然而,c-CO 2 RR 无法与 Ag 上的 HER 竞争。实验上,观察到的主要产物是 H 2 ,并产生少量 CO。通过具有明确传质特性的旋转圆柱电极电池,我们得出结论,尽管甲醇溶剂表现出较低的 HER 活性,但 HER 仍然优于 c-CO 2 RR。 我们的工作表明,甲醇盐是 NH 3 的潜在替代捕获剂,用于直接还原捕获的 CO 2 ,尽管催化剂设计方面存在挑战,特别是在降低 c-CO 的起始潜力方面 2 RR能否超越HER,还有待解决。
更新日期:2024-07-23
中文翻译:

捕获的 CO2 在银催化剂上的电还原:捕获剂和质子源的影响
在碳再利用的背景下,捕获的 CO 2 (c-CO 2 RR) 的直接电还原似乎是一种有吸引力的方法,因为它避免了能源成本高昂的 CO 2 来自捕获代理。在此过程中,CO 2 从其捕获形式直接还原。在这里,我们结合理论和实验研究了捕获剂和质子源对该反应的影响。具体来说,我们考虑银电催化剂上的甲醇盐捕获的 CO 2 、NH 3 捕获的 CO 2 和碳酸氢盐。我们表明,质子源在质子电还原、游离 CO 2 和捕获的 CO 2 化学反应的相互作用中起着关键作用。我们的密度泛函理论计算(包括电势的影响)表明,具有较小 pK a 的质子源提高了 c-CO 2 RR 的反应性,同时也提高了对银表面的析氢反应(HER)。由于c-CO 2 RR需要额外的化学质子化步骤,因此质子源的影响比HER的影响更强。然而,c-CO 2 RR 无法与 Ag 上的 HER 竞争。实验上,观察到的主要产物是 H 2 ,并产生少量 CO。通过具有明确传质特性的旋转圆柱电极电池,我们得出结论,尽管甲醇溶剂表现出较低的 HER 活性,但 HER 仍然优于 c-CO 2 RR。 我们的工作表明,甲醇盐是 NH 3 的潜在替代捕获剂,用于直接还原捕获的 CO 2 ,尽管催化剂设计方面存在挑战,特别是在降低 c-CO 的起始潜力方面 2 RR能否超越HER,还有待解决。












































 京公网安备 11010802027423号
京公网安备 11010802027423号