当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-Temperature Methane Combustion Using Ozone over Coβ Catalyst
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-20 , DOI: 10.1021/jacs.4c05967 Shunsaku Yasumura 1 , Ken Nagai 2 , Shinta Miyazaki 2 , Yucheng Qian 2 , Duotian Chen 2 , Takashi Toyao 2 , Yuichi Kamiya 3 , Ken-Ichi Shimizu 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-20 , DOI: 10.1021/jacs.4c05967 Shunsaku Yasumura 1 , Ken Nagai 2 , Shinta Miyazaki 2 , Yucheng Qian 2 , Duotian Chen 2 , Takashi Toyao 2 , Yuichi Kamiya 3 , Ken-Ichi Shimizu 2
Affiliation
|
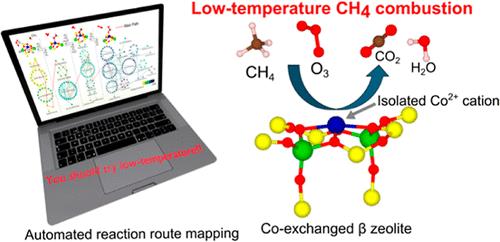
Catalytic methane (CH4) combustion is a promising approach to reducing the release of unburned methane in exhaust gas. Here, we report Co-exchanged β zeolite (Coβ) as an efficient catalyst for CH4 combustion using O3. A series of ion-exchanged β zeolites (Co, Ni, Mn, Fe, and Pd) are subjected to the catalytic test, and Coβ exhibits a superior performance in a low-temperature region (<100 °C). The results of X-ray absorption spectroscopy (XAS) and catalytic tests for Coβ with different Co loadings indicate the isolated Co species is the plausible active site. The reaction mechanism of CH4 combustion over the isolated Co2+ cation is theoretically investigated by the single-component artificial force-induced reaction (SC-AFIR) method to thoroughly search for possible reaction routes. The resulting path toward CO2 formation shows an activation energy of 73 kJ/mol for the rate-determining step and an exothermicity of 1025 kJ/mol, which supports the experimental results. During a long-term catalytic test for 160 h without external heating, the CH4 conversion gradually decreases from 80 to 40%, but the conversion fully recovers after dehydration at 500 °C (0.5 h). The copresence of H2O and CO exhibits a negative impact on the catalytic activity, while NO and SO2 do not markedly change the catalytic activity.
中文翻译:

Coβ 催化剂上使用臭氧进行低温甲烷燃烧
催化甲烷(CH 4 )燃烧是减少废气中未燃烧甲烷释放的一种有前景的方法。在这里,我们报道了Co-交换β沸石(Coβ)作为使用O 3进行CH 4燃烧的有效催化剂。对一系列离子交换β沸石(Co、Ni、Mn、Fe和Pd)进行了催化测试,Coβ在低温区表现出优异的性能(<100 id=222>4燃烧优于通过单组分人工力诱导反应(SC-AFIR)方法对分离的Co 2+阳离子进行了理论上的研究,以彻底寻找可能的反应路线,由此产生的CO 2形成路径显示出73 kJ/mol的活化能。速率决定步骤和1025 kJ/mol的放热,支持了实验结果。在没有外部加热的160小时的长期催化测试中,CH 4转化率逐渐从80%下降到40%,但转化率完全恢复。 500℃脱水(0.5h)后,H 2 O和CO的共存对催化活性产生负面影响,而NO和SO 2不会显着改变催化活性。
更新日期:2024-07-20
中文翻译:

Coβ 催化剂上使用臭氧进行低温甲烷燃烧
催化甲烷(CH 4 )燃烧是减少废气中未燃烧甲烷释放的一种有前景的方法。在这里,我们报道了Co-交换β沸石(Coβ)作为使用O 3进行CH 4燃烧的有效催化剂。对一系列离子交换β沸石(Co、Ni、Mn、Fe和Pd)进行了催化测试,Coβ在低温区表现出优异的性能(<100 id=222>4燃烧优于通过单组分人工力诱导反应(SC-AFIR)方法对分离的Co 2+阳离子进行了理论上的研究,以彻底寻找可能的反应路线,由此产生的CO 2形成路径显示出73 kJ/mol的活化能。速率决定步骤和1025 kJ/mol的放热,支持了实验结果。在没有外部加热的160小时的长期催化测试中,CH 4转化率逐渐从80%下降到40%,但转化率完全恢复。 500℃脱水(0.5h)后,H 2 O和CO的共存对催化活性产生负面影响,而NO和SO 2不会显着改变催化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号