当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Covalent Interactions of Anthocyanins with Proteins: Activity-Based Protein Profiling of Cyanidin-3-O-glucoside
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.jafc.4c03869 Jun Hu 1, 2 , Tingxin Yu 2 , Kuanchen Huang 2 , Chujie Liang 1 , Yue Li 2 , Xusheng Li 2 , Jianxia Sun 1 , Weibin Bai 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.jafc.4c03869 Jun Hu 1, 2 , Tingxin Yu 2 , Kuanchen Huang 2 , Chujie Liang 1 , Yue Li 2 , Xusheng Li 2 , Jianxia Sun 1 , Weibin Bai 2
Affiliation
|
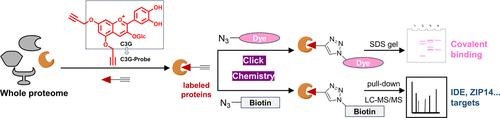
Anthocyanins are common natural pigments with a variety of physiological activities. Traditional perspectives attribute their molecular mechanism to noncovalent interactions influencing signaling pathways. However, this ignores the nature of its benzopyrylium skeleton, which readily reacts with the electron-rich groups of proteins. Here, we modified cyanidin-3-O-glucoside (C3G) via activity-based protein profiling technology by our previous synthesis route and prepared the covalent binding probe (C3G-Probe) and the noncovalent photoaffinity probe (C3G-Diazirine). The properties of C3G′s covalent binding to proteins were also discovered by comparing the labeling of the two probes to the whole HepG2 cell proteome. We further explored its target proteins and enriched pathways in HepG2 and HeLa cells. Western blot analysis further confirmed the covalent binding of C3G to four target proteins: insulin-degrading enzyme, metal cation symporter ZIP14, spermatid perinuclear RNA-binding protein, and Cystatin-B. Pathway analysis showed that covalent targets of C3G were concentrated in metabolic pathways and several ribonucleoprotein complexes that were also coenriched. The results of this study provide new insights into the interaction of the naturally active molecule C3G with proteins.
中文翻译:

花青素与蛋白质的共价相互作用:Cyanidin-3-O-glucoside 基于活性的蛋白质分析
花青素是常见的天然色素,具有多种生理活性。传统观点将其分子机制归因于影响信号通路的非共价相互作用。然而,这忽略了其苯并吡喃鎓骨架的性质,该骨架很容易与富含电子的蛋白质基团发生反应。在这里,我们通过基于活性的蛋白质分析技术,按照我们之前的合成路线对花青素-3- O-葡萄糖苷(C3G)进行修饰,并制备了共价结合探针(C3G-Probe)和非共价光亲和探针(C3G-Diazirine)。通过比较两种探针与整个 HepG2 细胞蛋白质组的标记,还发现了 C3G 与蛋白质共价结合的特性。我们进一步探索了其在 HepG2 和 HeLa 细胞中的靶蛋白和富集通路。 Western blot分析进一步证实了C3G与四种靶蛋白的共价结合:胰岛素降解酶、金属阳离子同向转运蛋白ZIP14、精子细胞核周RNA结合蛋白和半胱氨酸蛋白酶抑制剂-B。通路分析表明,C3G 的共价靶标集中在代谢通路中,并且一些核糖核蛋白复合物也被共富集。这项研究的结果为天然活性分子 C3G 与蛋白质的相互作用提供了新的见解。
更新日期:2024-07-22
中文翻译:

花青素与蛋白质的共价相互作用:Cyanidin-3-O-glucoside 基于活性的蛋白质分析
花青素是常见的天然色素,具有多种生理活性。传统观点将其分子机制归因于影响信号通路的非共价相互作用。然而,这忽略了其苯并吡喃鎓骨架的性质,该骨架很容易与富含电子的蛋白质基团发生反应。在这里,我们通过基于活性的蛋白质分析技术,按照我们之前的合成路线对花青素-3- O-葡萄糖苷(C3G)进行修饰,并制备了共价结合探针(C3G-Probe)和非共价光亲和探针(C3G-Diazirine)。通过比较两种探针与整个 HepG2 细胞蛋白质组的标记,还发现了 C3G 与蛋白质共价结合的特性。我们进一步探索了其在 HepG2 和 HeLa 细胞中的靶蛋白和富集通路。 Western blot分析进一步证实了C3G与四种靶蛋白的共价结合:胰岛素降解酶、金属阳离子同向转运蛋白ZIP14、精子细胞核周RNA结合蛋白和半胱氨酸蛋白酶抑制剂-B。通路分析表明,C3G 的共价靶标集中在代谢通路中,并且一些核糖核蛋白复合物也被共富集。这项研究的结果为天然活性分子 C3G 与蛋白质的相互作用提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号