当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Agglomeration and Microscopic Molecular Exchange Mechanism in Antisolvent Crystallization
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.cgd.4c00456 Chunlei Qin 1 , Peng Yang 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2024-07-22 , DOI: 10.1021/acs.cgd.4c00456 Chunlei Qin 1 , Peng Yang 1
Affiliation
|
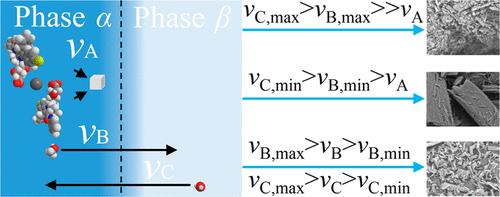
Agglomeration is a significant challenge in antisolvent crystallization, especially when it comes to crystallizing insoluble drugs. This study aimed to address this issue by taking a unique, dynamic molecular perspective and proposing a solution based on microscopic molecular exchange. Atorvastatin calcium, a widely prescribed medication for lowering blood lipids, was used as a case for antisolvent crystallization, which is also prone to agglomeration. Kinetic experiments revealed the rapid exchange between solvent and antisolvent, leading to precipitation occurring within a mere 0.0333 s. Thermodynamic investigations further showed that each droplet had the potential to generate an astonishing equivalent nucleation rate of up to 5 billion nuclei per second. The rapid precipitation and small nucleation region, coupled with a large number of nuclei, worsened the crystal agglomeration problem. However, it was found that this molecular exchange process can be slowed by the solvent composition. Specifically, when the methanol mole fraction in the crystallization system reached approximately 50%, the equivalent nucleation rate decreased significantly to about 100 million per second. Armed with this crucial finding, a strategy was devised to overcome agglomeration by simultaneously adding the solution and antisolvent to maintain a lower exchange rate, which effectively avoided agglomeration and promoted the purification of atorvastatin calcium.
中文翻译:

反溶剂结晶中的晶体团聚和微观分子交换机制
团聚是反溶剂结晶中的一个重大挑战,特别是在结晶不溶性药物时。本研究旨在通过采取独特的动态分子视角并提出基于微观分子交换的解决方案来解决这个问题。阿托伐他汀钙是一种广泛使用的降血脂药物,被用作反溶剂结晶的案例,该结晶也容易结块。动力学实验揭示了溶剂和反溶剂之间的快速交换,导致沉淀在仅仅 0.0333 秒内发生。热力学研究进一步表明,每个液滴都有可能产生高达每秒 50 亿个核的惊人的等效成核率。快速的沉淀和较小的成核区域,再加上大量的晶核,加剧了晶体团聚问题。然而,人们发现溶剂组成会减慢这种分子交换过程。具体来说,当结晶体系中的甲醇摩尔分数达到约50%时,等效成核速率显着下降至约1亿个/秒。基于这一重要发现,我们设计了一种克服团聚的策略,通过同时添加溶液和反溶剂以保持较低的交换率,有效地避免团聚并促进阿托伐他汀钙的纯化。
更新日期:2024-07-22
中文翻译:

反溶剂结晶中的晶体团聚和微观分子交换机制
团聚是反溶剂结晶中的一个重大挑战,特别是在结晶不溶性药物时。本研究旨在通过采取独特的动态分子视角并提出基于微观分子交换的解决方案来解决这个问题。阿托伐他汀钙是一种广泛使用的降血脂药物,被用作反溶剂结晶的案例,该结晶也容易结块。动力学实验揭示了溶剂和反溶剂之间的快速交换,导致沉淀在仅仅 0.0333 秒内发生。热力学研究进一步表明,每个液滴都有可能产生高达每秒 50 亿个核的惊人的等效成核率。快速的沉淀和较小的成核区域,再加上大量的晶核,加剧了晶体团聚问题。然而,人们发现溶剂组成会减慢这种分子交换过程。具体来说,当结晶体系中的甲醇摩尔分数达到约50%时,等效成核速率显着下降至约1亿个/秒。基于这一重要发现,我们设计了一种克服团聚的策略,通过同时添加溶液和反溶剂以保持较低的交换率,有效地避免团聚并促进阿托伐他汀钙的纯化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号