Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A programmable and automated optical electrowetting-on-dielectric (oEWOD) driven platform for massively parallel and sequential processing of single cell assay operations
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4lc00245h Lawrence G Welch 1 , Jasper Estranero 1 , Panagiotis Tourlomousis 2 , Robert C R Wootton 1 , Valentin Radu 1 , Carlos González-Fernández 1 , Tim J Puchtler 1 , Claire M Murzeau 1 , Nele M G Dieckmann 1 , Aya Shibahara 1 , Brooke W Longbottom 1 , Clare E Bryant 2 , Emma L Talbot 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4lc00245h Lawrence G Welch 1 , Jasper Estranero 1 , Panagiotis Tourlomousis 2 , Robert C R Wootton 1 , Valentin Radu 1 , Carlos González-Fernández 1 , Tim J Puchtler 1 , Claire M Murzeau 1 , Nele M G Dieckmann 1 , Aya Shibahara 1 , Brooke W Longbottom 1 , Clare E Bryant 2 , Emma L Talbot 1
Affiliation
|
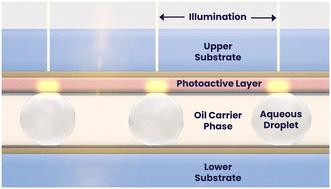
Recently, there has been an increasing emphasis on single cell profiling for high-throughput screening workflows in drug discovery and life sciences research. However, the biology underpinning these screens is often complex and is insufficiently addressed by singleplex assay screens. Traditional single cell screening technologies have created powerful sets of ‘omic data that allow users to bioinformatically infer biological function, but have as of yet not empowered direct functional analysis at the level of each individual cell. Consequently, screening campaigns often require multiple secondary screens leading to laborious, time-consuming and expensive workflows in which attrition points may not be queried until late in the process. We describe a platform that harnesses droplet microfluidics and optical electrowetting-on-dielectric (oEWOD) to perform highly-controlled sequential and multiplexed single cell assays in massively parallelised workflows to enable complex cell profiling during screening. Soluble reagents or objects, such as cells or assay beads, are encapsulated into droplets of media in fluorous oil and are actively filtered based on size and optical features ensuring only desirable droplets (e.g. single cell droplets) are retained for analysis, thereby overcoming the Poisson probability distribution. Droplets are stored in an array on a temperature-controlled chip and the history of individual droplets is logged from the point of filter until completion of the workflow. On chip, droplets are subject to an automated and flexible suite of operations including the merging of sample droplets and the fluorescent acquisition of assay readouts to enable complex sequential assay workflows. To demonstrate the broad utility of the platform, we present examples of single-cell functional workflows for various applications such as antibody discovery, infectious disease, and cell and gene therapy.
中文翻译:

可编程和自动化的电介质光学电润湿 (oEWOD) 驱动平台,用于单细胞检测操作的大规模并行和顺序处理
最近,药物发现和生命科学研究中的高通量筛选工作流程的单细胞分析越来越受到重视。然而,支撑这些筛选的生物学通常很复杂,单重分析筛选无法充分解决这一问题。传统的单细胞筛选技术已经创建了强大的组学数据集,允许用户通过生物信息学推断生物功能,但迄今为止尚未能够在每个单独细胞的水平上进行直接功能分析。因此,筛选活动通常需要多个辅助筛选,从而导致工作流程费力、耗时且昂贵,其中可能直到流程后期才查询到损耗点。我们描述了一个平台,该平台利用液滴微流体和光学电介质电润湿(oEWOD)在大规模并行工作流程中执行高度控制的顺序和多重单细胞测定,从而在筛选过程中实现复杂的细胞分析。可溶性试剂或物体(例如细胞或分析珠)被封装在氟油中的介质液滴中,并根据尺寸和光学特征进行主动过滤,确保仅保留所需的液滴(例如单细胞液滴)进行分析,从而克服泊松效应概率分布。液滴存储在温控芯片上的阵列中,并记录从过滤点到工作流程完成的各个液滴的历史记录。在芯片上,液滴受到一套自动化且灵活的操作,包括样品液滴的合并和测定读数的荧光采集,以实现复杂的顺序测定工作流程。 为了展示该平台的广泛实用性,我们提供了用于抗体发现、传染病以及细胞和基因治疗等各种应用的单细胞功能工作流程的示例。
更新日期:2024-07-22
中文翻译:

可编程和自动化的电介质光学电润湿 (oEWOD) 驱动平台,用于单细胞检测操作的大规模并行和顺序处理
最近,药物发现和生命科学研究中的高通量筛选工作流程的单细胞分析越来越受到重视。然而,支撑这些筛选的生物学通常很复杂,单重分析筛选无法充分解决这一问题。传统的单细胞筛选技术已经创建了强大的组学数据集,允许用户通过生物信息学推断生物功能,但迄今为止尚未能够在每个单独细胞的水平上进行直接功能分析。因此,筛选活动通常需要多个辅助筛选,从而导致工作流程费力、耗时且昂贵,其中可能直到流程后期才查询到损耗点。我们描述了一个平台,该平台利用液滴微流体和光学电介质电润湿(oEWOD)在大规模并行工作流程中执行高度控制的顺序和多重单细胞测定,从而在筛选过程中实现复杂的细胞分析。可溶性试剂或物体(例如细胞或分析珠)被封装在氟油中的介质液滴中,并根据尺寸和光学特征进行主动过滤,确保仅保留所需的液滴(例如单细胞液滴)进行分析,从而克服泊松效应概率分布。液滴存储在温控芯片上的阵列中,并记录从过滤点到工作流程完成的各个液滴的历史记录。在芯片上,液滴受到一套自动化且灵活的操作,包括样品液滴的合并和测定读数的荧光采集,以实现复杂的顺序测定工作流程。 为了展示该平台的广泛实用性,我们提供了用于抗体发现、传染病以及细胞和基因治疗等各种应用的单细胞功能工作流程的示例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号