Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of humic acid by UV/PMS: process comparison, influencing factors, and degradation mechanism
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra04328f Qingchao Shen 1, 2 , Xiaosan Song 1, 2 , Jishuo Fan 1, 2 , Cheng Chen 1, 2 , Zili Guo 1, 2
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra04328f Qingchao Shen 1, 2 , Xiaosan Song 1, 2 , Jishuo Fan 1, 2 , Cheng Chen 1, 2 , Zili Guo 1, 2
Affiliation
|
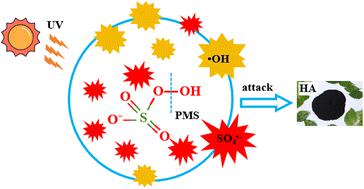
In natural water bodies, humic acid (HA), generated during the chlorination disinfection process at water treatment plants, can produce halogenated disinfection by-products, increasing the risk to drinking water safety and posing a threat to human health. Effectively removing HA from natural waters is a critical focus of environmental research. This study established a synergistic ultraviolet/peroxymonosulfate (UV/PMS) system to remove HA from water. It compared the efficacy of various UV/advanced oxidation processes (AOPs) on HA degradation, and assessed the influence of different water sources, initial pH, oxidant concentration, and anions (HCO3−, Cl−, NO3−) on HA degradation. The degradation mechanism of HA by the UV/PMS process was also investigated. Results showed that under the conditions of 3 mmol L−1 PMS concentration, 10 mg L−1 HA concentration, initial solution pH of 7, and a reaction time of 240 minutes, the mineralization rate of HA by UV/PMS reached 94.15%. The pseudo-first-order kinetic constant (kobs) was 0.01034 and the single-electric energy (EE/O) was 0.0157 kW h m−3, indicating superior HA removal efficiency compared to other systems. Common anions (HCO3−, Cl−, NO3−) in water were found to inhibit the degradation of HA, and acidic conditions were more conducive to HA removal, with the optimal pH being 3. Free radical quenching experiments showed that both sulfate radical (SO4−˙) and hydroxyl radical (˙OH) radicals were involved in HA degradation, with SO4−˙ being the primary oxidant and ˙OH as the auxiliary species. Analyses using 3D-excitation-emission matrix (EEM), parallel factor analysis (PARAFAC), specific fluorescence index, and absorbance demonstrated that UV/PMS technology could effectively degrade HA in water. This study provides theoretical references for further research on the removal of HA and other organic substances using UV/PMS technology.
中文翻译:

UV/PMS 降解腐殖酸:工艺比较、影响因素和降解机制
在天然水体中,水处理厂氯化消毒过程中产生的腐殖酸(HA)会产生卤化消毒副产物,增加饮用水安全风险,对人体健康构成威胁。有效去除天然水中的透明质酸是环境研究的一个重点。本研究建立了一种协同紫外线/过一硫酸盐 (UV/PMS) 系统来去除水中的 HA。比较了各种紫外/高级氧化工艺(AOP)对HA降解的效果,并评估了不同水源、初始pH、氧化剂浓度和阴离子(HCO 3 - 、Cl - 、NO 3 - )对HA降解的影响。还研究了 UV/PMS 过程对 HA 的降解机制。结果表明,在PMS浓度3 mmol L -1 、HA浓度10 mg L -1 、初始溶液pH为7、反应时间240 min条件下,UV/PMS对HA的矿化率达到94.15%。准一级动力学常数( k obs )为0.01034,单次电能(EE/O)为0.0157 kW hm -3 ,表明与其他系统相比具有优异的HA去除效率。 研究发现水中常见阴离子(HCO 3 - 、Cl - 、NO 3 - )可抑制HA的降解,酸性条件更有利于HA去除,最佳pH为3。自由基猝灭实验表明,硫酸根自由基(SO 4 − ˙)和羟基自由基(˙OH)参与HA降解,其中SO 4 − ˙为主要氧化剂,˙OH为辅助氧化剂。使用 3D 激发发射矩阵 (EEM)、平行因子分析 (PARAFAC)、特定荧光指数和吸光度进行的分析表明,UV/PMS 技术可以有效降解水中的 HA。本研究为进一步研究利用UV/PMS技术去除HA等有机物提供理论参考。
更新日期:2024-07-22
中文翻译:

UV/PMS 降解腐殖酸:工艺比较、影响因素和降解机制
在天然水体中,水处理厂氯化消毒过程中产生的腐殖酸(HA)会产生卤化消毒副产物,增加饮用水安全风险,对人体健康构成威胁。有效去除天然水中的透明质酸是环境研究的一个重点。本研究建立了一种协同紫外线/过一硫酸盐 (UV/PMS) 系统来去除水中的 HA。比较了各种紫外/高级氧化工艺(AOP)对HA降解的效果,并评估了不同水源、初始pH、氧化剂浓度和阴离子(HCO 3 - 、Cl - 、NO 3 - )对HA降解的影响。还研究了 UV/PMS 过程对 HA 的降解机制。结果表明,在PMS浓度3 mmol L -1 、HA浓度10 mg L -1 、初始溶液pH为7、反应时间240 min条件下,UV/PMS对HA的矿化率达到94.15%。准一级动力学常数( k obs )为0.01034,单次电能(EE/O)为0.0157 kW hm -3 ,表明与其他系统相比具有优异的HA去除效率。 研究发现水中常见阴离子(HCO 3 - 、Cl - 、NO 3 - )可抑制HA的降解,酸性条件更有利于HA去除,最佳pH为3。自由基猝灭实验表明,硫酸根自由基(SO 4 − ˙)和羟基自由基(˙OH)参与HA降解,其中SO 4 − ˙为主要氧化剂,˙OH为辅助氧化剂。使用 3D 激发发射矩阵 (EEM)、平行因子分析 (PARAFAC)、特定荧光指数和吸光度进行的分析表明,UV/PMS 技术可以有效降解水中的 HA。本研究为进一步研究利用UV/PMS技术去除HA等有机物提供理论参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号