Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Harnessing the power of thermosensitive liposomes with gold nanoprisms and silica for controlled drug delivery in combined chemotherapy and phototherapy
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra03359k Marta Rubio-Camacho 1 , Carlos Cuestas-Ayllón 2 , Beatriz Torres-Herrero 2 , María José Martínez-Tomé 1 , Jesús M. de la Fuente 2 , C. Reyes Mateo 1
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra03359k Marta Rubio-Camacho 1 , Carlos Cuestas-Ayllón 2 , Beatriz Torres-Herrero 2 , María José Martínez-Tomé 1 , Jesús M. de la Fuente 2 , C. Reyes Mateo 1
Affiliation
|
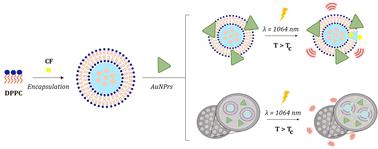
In recent years, the scientific community has tried to address the treatment of complex diseases such as cancer in a more appropriate and promising way. Regarding this and benefiting from the unique optical properties of gold nanoprisms (AuNPRs), the physicochemical properties of thermosensitive liposomes (TSLs), and the tunable drug encapsulation and release properties of silica nanoparticles (BioSi@NPs), this study has developed two nanoformulations. These nanoformulations have the potential to integrate chemotherapy and photothermal therapy within a single entity. Once their components were synthesized and characterized separately, two strategies were taken in order to develop these multifunctional nanoformulations: (1) covalent binding of AuNPRs to TSLs and (2) co-encapsulation of both components within BioSi@NPs, without modifying the optical and physicochemical properties of AuNPRs and TSLs. Finally, the suitability of both nanoformulations to carry and release hydrophilic drugs when triggered by a 1064 nm NIR laser has been explored by using the fluorescent probe 5(6)-carboxyfluorescein (CF) as a hydrophilic drug model. Different laser power and time of exposure were also tested evidencing that hydrophilic drugs were only released from TSLs in the presence of AuNPRs and that the drug release profile was dependent on the type of nanoformulation and irradiation conditions used. In conclusion, these multifunctional nanoformulations exhibit promising potential for controlled drug delivery in combined chemotherapy and phototherapy, with the capability to precisely control the release kinetics based on specific therapeutic needs.
中文翻译:

利用金纳米棱柱和二氧化硅热敏脂质体的力量在联合化疗和光疗中控制药物输送
近年来,科学界试图以更合适、更有前景的方式解决癌症等复杂疾病的治疗问题。为此,受益于金纳米棱柱(AuNPRs)独特的光学特性、热敏脂质体(TSLs)的理化特性以及二氧化硅纳米粒子(BioSi@NPs)可调节的药物封装和释放特性,本研究开发了两种纳米制剂。这些纳米制剂有可能将化疗和光热疗法整合到一个实体中。一旦它们的组分被分别合成和表征,就采取了两种策略来开发这些多功能纳米制剂:(1) AuNPR 与 TSL 的共价结合;(2) 将两种组分共封装在 BioSi@NP 内,而不改变光学和光学性质。 AuNPR 和 TSL 的物理化学性质。最后,通过使用荧光探针 5(6)-羧基荧光素 (CF) 作为亲水性药物模型,探索了两种纳米制剂在 1064 nm NIR 激光触发时携带和释放亲水性药物的适用性。还测试了不同的激光功率和暴露时间,证明亲水性药物仅在 AuNPR 存在的情况下从 TSL 中释放,并且药物释放曲线取决于纳米制剂的类型和所使用的照射条件。总之,这些多功能纳米制剂在联合化疗和光疗中的受控药物递送方面表现出良好的潜力,能够根据特定的治疗需求精确控制释放动力学。
更新日期:2024-07-22
中文翻译:

利用金纳米棱柱和二氧化硅热敏脂质体的力量在联合化疗和光疗中控制药物输送
近年来,科学界试图以更合适、更有前景的方式解决癌症等复杂疾病的治疗问题。为此,受益于金纳米棱柱(AuNPRs)独特的光学特性、热敏脂质体(TSLs)的理化特性以及二氧化硅纳米粒子(BioSi@NPs)可调节的药物封装和释放特性,本研究开发了两种纳米制剂。这些纳米制剂有可能将化疗和光热疗法整合到一个实体中。一旦它们的组分被分别合成和表征,就采取了两种策略来开发这些多功能纳米制剂:(1) AuNPR 与 TSL 的共价结合;(2) 将两种组分共封装在 BioSi@NP 内,而不改变光学和光学性质。 AuNPR 和 TSL 的物理化学性质。最后,通过使用荧光探针 5(6)-羧基荧光素 (CF) 作为亲水性药物模型,探索了两种纳米制剂在 1064 nm NIR 激光触发时携带和释放亲水性药物的适用性。还测试了不同的激光功率和暴露时间,证明亲水性药物仅在 AuNPR 存在的情况下从 TSL 中释放,并且药物释放曲线取决于纳米制剂的类型和所使用的照射条件。总之,这些多功能纳米制剂在联合化疗和光疗中的受控药物递送方面表现出良好的潜力,能够根据特定的治疗需求精确控制释放动力学。












































 京公网安备 11010802027423号
京公网安备 11010802027423号