Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zn(OTf)2-catalyzed intra- and intermolecular selenofunctionalization of alkenes under mild conditions
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra04266b Cong Qi 1 , Zhaogong Lu 1 , Yuyang Gu 2 , Xiaofeng Bao 1 , Biao Xiong 1 , Gong-Qing Liu 1
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-22 , DOI: 10.1039/d4ra04266b Cong Qi 1 , Zhaogong Lu 1 , Yuyang Gu 2 , Xiaofeng Bao 1 , Biao Xiong 1 , Gong-Qing Liu 1
Affiliation
|
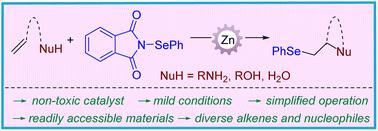
Zn(OTf)2-catalyzed intra- and intermolecular selenofunctionalization of alkenes was achieved with electrophilic N-phenylselenophthalimide. This method provides straightforward and efficient access to various seleno-substituted heterocycles and vicinal Se heteroatom-disubstituted molecules under mild conditions. This reaction is compatible with various substrates/functional groups, and preliminary studies on the reaction mechanistic were also conducted.
中文翻译:

温和条件下 Zn(OTf)2 催化烯烃分子内和分子间硒功能化
Zn(OTf) 2催化的烯烃分子内和分子间硒基官能化是用亲电子N-苯基硒代邻苯二甲酰亚胺实现的。该方法提供了在温和条件下直接有效地获取各种硒代杂环和邻位硒杂原子二取代分子的方法。该反应与各种底物/官能团相容,并对反应机理进行了初步研究。
更新日期:2024-07-22
中文翻译:

温和条件下 Zn(OTf)2 催化烯烃分子内和分子间硒功能化
Zn(OTf) 2催化的烯烃分子内和分子间硒基官能化是用亲电子N-苯基硒代邻苯二甲酰亚胺实现的。该方法提供了在温和条件下直接有效地获取各种硒代杂环和邻位硒杂原子二取代分子的方法。该反应与各种底物/官能团相容,并对反应机理进行了初步研究。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号