当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Reactivity through Spin Manipulation: Steric Bulkiness of Peroxocobalt(III) Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-20 , DOI: 10.1021/jacs.4c03211 Seonghan Kim 1, 2 , Yuri Lee 1 , Guilherme L Tripodi 3 , Jana Roithová 3 , Sunggi Lee 2 , Jaeheung Cho 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-20 , DOI: 10.1021/jacs.4c03211 Seonghan Kim 1, 2 , Yuri Lee 1 , Guilherme L Tripodi 3 , Jana Roithová 3 , Sunggi Lee 2 , Jaeheung Cho 1
Affiliation
|
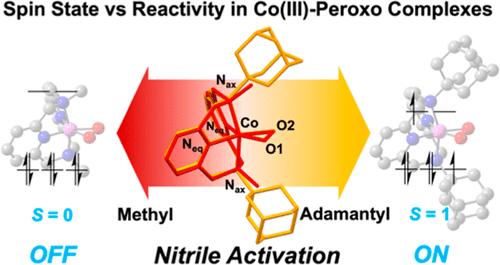
The intrinsic relationship between spin states and reactivity in peroxocobalt(III) complexes was investigated, specifically focusing on the influence of steric modulation on supporting ligands. Together with the previously reported [CoIII(TBDAP)(O2)]+ (2Tb), which exhibits spin crossover characteristics, two peroxocobalt(III) complexes, [CoIII(MDAP)(O2)]+ (2Me) and [CoIII(ADDAP)(O2)]+ (2Ad), bearing pyridinophane ligands with distinct N-substituents such as methyl and adamantyl groups, were synthesized and characterized. By manipulating the steric bulkiness of the N-substituents, control of spin states in peroxocobalt(III) complexes was demonstrated through various physicochemical analyses. Notably, 2Ad oxidized the nitriles to generate hydroximatocobalt(III) complexes, while 2Me displayed an inability for such oxidation reactions. Furthermore, both 2Ad and 2Tb exhibited similarities in spectroscopic and geometric features, demonstrating spin crossover behavior between S = 0 and S = 1. The steric bulkiness of the adamantyl and tert-butyl group on the axial amines was attributed to inducing a weak ligand field on the cobalt(III) center. Thus, 2Ad and 2Tb are an S = 1 state under the reaction conditions. In contrast, the less bulky methyl group on the amines of 2Me resulted in an S = 0 state. The redox potential of the peroxocobalt(III) complexes was also influenced by the ligand field arising from the steric bulkiness of the N-substituents in the order of 2Me (−0.01 V) < 2Tb (0.29 V) = 2Ad (0.29 V). Theoretical calculations using DFT supported the experimental observations, providing insights into the electronic structure and emphasizing the importance of the spin state of peroxocobalt(III) complexes in nitrile activation.
中文翻译:

通过自旋操纵控制反应性:过氧钴 (III) 配合物的空间体积
研究了过氧钴(III)配合物中自旋态和反应性之间的内在关系,特别关注空间调节对支持配体的影响。与先前报道的具有自旋交叉特性的[Co III (TBDAP)(O 2 )] + ( 2 Tb )一起,两个过氧钴(III)配合物,[Co III (MDAP)(O 2 )] + ( 2 Me )和[Co III (ADDAP)(O 2 )] + ( 2 Ad ),带有带有不同N-取代基(如甲基和金刚烷基)的吡啶芬配体,进行了合成和表征。通过操纵N-取代基的空间体积,通过各种物理化学分析证明了对过氧钴(III)络合物中自旋态的控制。值得注意的是, 2 Ad氧化腈生成羟肟钴(III)络合物,而2 Me 则无法进行此类氧化反应。此外, 2 Ad和2 Tb在光谱和几何特征上表现出相似性,证明了S = 0 和S = 1 之间的自旋交叉行为。轴向胺上金刚烷基和叔丁基的空间体积归因于诱导弱钴(III)中心的配体场。 因此, 2 Ad和2 Tb在反应条件下是S =1状态。相反, 2 Me的胺上体积较小的甲基导致S = 0 状态。过氧钴(III)配合物的氧化还原电位还受到N-取代基的空间体积产生的配体场的影响,其顺序为2 Me (-0.01 V) < 2 Tb (0.29 V) = 2 Ad (0.29五)。使用 DFT 的理论计算支持了实验观察,提供了对电子结构的见解,并强调了过氧钴 (III) 配合物的自旋态在腈活化中的重要性。
更新日期:2024-07-20
中文翻译:

通过自旋操纵控制反应性:过氧钴 (III) 配合物的空间体积
研究了过氧钴(III)配合物中自旋态和反应性之间的内在关系,特别关注空间调节对支持配体的影响。与先前报道的具有自旋交叉特性的[Co III (TBDAP)(O 2 )] + ( 2 Tb )一起,两个过氧钴(III)配合物,[Co III (MDAP)(O 2 )] + ( 2 Me )和[Co III (ADDAP)(O 2 )] + ( 2 Ad ),带有带有不同N-取代基(如甲基和金刚烷基)的吡啶芬配体,进行了合成和表征。通过操纵N-取代基的空间体积,通过各种物理化学分析证明了对过氧钴(III)络合物中自旋态的控制。值得注意的是, 2 Ad氧化腈生成羟肟钴(III)络合物,而2 Me 则无法进行此类氧化反应。此外, 2 Ad和2 Tb在光谱和几何特征上表现出相似性,证明了S = 0 和S = 1 之间的自旋交叉行为。轴向胺上金刚烷基和叔丁基的空间体积归因于诱导弱钴(III)中心的配体场。 因此, 2 Ad和2 Tb在反应条件下是S =1状态。相反, 2 Me的胺上体积较小的甲基导致S = 0 状态。过氧钴(III)配合物的氧化还原电位还受到N-取代基的空间体积产生的配体场的影响,其顺序为2 Me (-0.01 V) < 2 Tb (0.29 V) = 2 Ad (0.29五)。使用 DFT 的理论计算支持了实验观察,提供了对电子结构的见解,并强调了过氧钴 (III) 配合物的自旋态在腈活化中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号