当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiourea as a “Polar Hydrophobic” Hydrogen-Bonding Motif: Application to Highly Durable All-Underwater Adhesion
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-20 , DOI: 10.1021/jacs.4c07515 Kohei Kikkawa 1 , Yosuke Sumiya 2 , Kazuki Okazawa 2 , Kazunari Yoshizawa 2 , Yoshimitsu Itoh 1, 3 , Takuzo Aida 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-20 , DOI: 10.1021/jacs.4c07515 Kohei Kikkawa 1 , Yosuke Sumiya 2 , Kazuki Okazawa 2 , Kazunari Yoshizawa 2 , Yoshimitsu Itoh 1, 3 , Takuzo Aida 1, 4
Affiliation
|
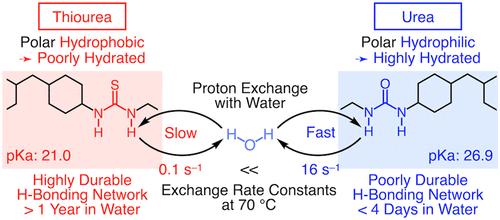
Here, we report that, in contrast to urea, thiourea functions as a “polar hydrophobic” hydrogen-bonding motif. Although thiourea is more acidic than urea, thiourea exchanges its N–H protons with water at a rate that is 160 times slower than that for urea at 70 °C. This suggests that thiourea is much less hydrated than urea in an aqueous environment. What led us to this interesting principle was the serendipitous finding that self-healable poly(ether thiourea) adhered strongly to wet glass surfaces. This discovery enabled us to develop an exceptionally durable all-underwater adhesive that can maintain large adhesive strength for over a year even in seawater, simply by mechanically mixing three water-insoluble liquid components on target surfaces. Because thiourea is hydrophobic, its hydrogen-bonding networks within the adhesive structure and at the adhesive–target interface are presumed to be dehydrated. For comparison, a reference adhesive using urea as a representative "polar hydrophilic" hydrogen-bonding motif was durable for less than 4 days in water. Highly durable all-underwater adhesives are needed in various fields of marine engineering and biomedical sciences, but their development has been a major challenge because a hydration layer that spontaneously forms in water always inhibits adhesion.
中文翻译:

硫脲作为“极性疏水”氢键基序:在高度耐用的全水下粘合中的应用
在这里,我们报告说,与尿素相反,硫脲起到“极性疏水”氢键基序的作用。尽管硫脲的酸性比尿素更强,但硫脲在 70 °C 时与水交换 N-H 质子的速率比尿素慢 160 倍。这表明在水性环境中硫脲的水合性比尿素低得多。使我们得出这个有趣原理的是一个偶然的发现,即自修复聚醚硫脲可以牢固地粘附在湿玻璃表面上。这一发现使我们能够开发出一种极其耐用的全水下粘合剂,只需在目标表面上机械混合三种不溶于水的液体成分,即使在海水中也能保持一年以上的高粘合强度。由于硫脲是疏水性的,因此其在粘合剂结构内和粘合剂-目标界面处的氢键网络被认为是脱水的。为了进行比较,使用尿素作为代表性“极性亲水”氢键基序的参考粘合剂在水中的耐用时间不到 4 天。海洋工程和生物医学的各个领域都需要高度耐用的全水下粘合剂,但它们的开发一直是一个重大挑战,因为在水中自发形成的水化层总是会抑制粘合。
更新日期:2024-07-20
中文翻译:

硫脲作为“极性疏水”氢键基序:在高度耐用的全水下粘合中的应用
在这里,我们报告说,与尿素相反,硫脲起到“极性疏水”氢键基序的作用。尽管硫脲的酸性比尿素更强,但硫脲在 70 °C 时与水交换 N-H 质子的速率比尿素慢 160 倍。这表明在水性环境中硫脲的水合性比尿素低得多。使我们得出这个有趣原理的是一个偶然的发现,即自修复聚醚硫脲可以牢固地粘附在湿玻璃表面上。这一发现使我们能够开发出一种极其耐用的全水下粘合剂,只需在目标表面上机械混合三种不溶于水的液体成分,即使在海水中也能保持一年以上的高粘合强度。由于硫脲是疏水性的,因此其在粘合剂结构内和粘合剂-目标界面处的氢键网络被认为是脱水的。为了进行比较,使用尿素作为代表性“极性亲水”氢键基序的参考粘合剂在水中的耐用时间不到 4 天。海洋工程和生物医学的各个领域都需要高度耐用的全水下粘合剂,但它们的开发一直是一个重大挑战,因为在水中自发形成的水化层总是会抑制粘合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号