当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate Characterization for Hydrolysis of Multiple Types of Aromatic Esters by Promiscuous Aminopeptidases
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-18 , DOI: 10.1021/acs.jafc.4c02053 Hang Ning 1 , Wen-Long Liu 1 , Qing-Yun Li 1, 2 , You-Yan Liu 1, 2 , Shi-Ting Huang 1 , Hai-Bo Liu 1 , Ai-Xing Tang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-18 , DOI: 10.1021/acs.jafc.4c02053 Hang Ning 1 , Wen-Long Liu 1 , Qing-Yun Li 1, 2 , You-Yan Liu 1, 2 , Shi-Ting Huang 1 , Hai-Bo Liu 1 , Ai-Xing Tang 1, 2
Affiliation
|
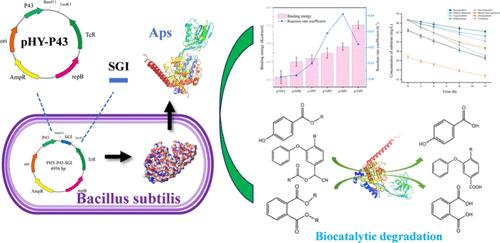
Synthetic aromatic esters, widely employed in agriculture, food, and chemical industries, have become emerging environmental pollutants due to their strong hydrophobicity and poor bioavailability. This study attempted to address this issue by extracellularly expressing the promiscuous aminopeptidase (Aps) from Pseudomonas aeruginosa GF31 in B. subtilis, achieving an impressive enzyme activity of 13.7 U/mg. Notably, we have demonstrated, for the first time, the Aps-mediated degradation of diverse aromatic esters, including but not limited to pyrethroids, phthalates, and parabens. A biochemical characterization of Aps reveals its esterase properties and a broader spectrum of substrate profiles. The degradation rates of p-nitrobenzene esters (p-NB) with different side chain structures vary under the action of Aps, showing a preference for substrates with relatively longer alkyl side chains. The structure-dependent degradability aligns well with the binding energies between Aps and p-NB. Molecular docking and enzyme–substrate interaction elucidate that hydrogen bonding, hydrophobic interactions, and π–π stacking collectively stabilize the enzyme–substrate conformation, promoting substrate hydrolysis. These findings provide new insights into the enzymatic degradation of aromatic ester pollutants, laying a foundation for the further development and modification of promiscuous enzymes.
中文翻译:

混杂氨基肽酶水解多种芳香酯的底物表征
合成芳香酯广泛应用于农业、食品和化学工业,但由于其强疏水性和较差的生物利用度,已成为新兴的环境污染物。本研究试图通过在枯草芽孢杆菌中细胞外表达来自铜绿假单胞菌GF31 的混杂氨肽酶 (Aps) 来解决这个问题,实现了 13.7 U/mg 的令人印象深刻的酶活性。值得注意的是,我们首次证明了 Aps 介导的多种芳香酯的降解,包括但不限于拟除虫菊酯、邻苯二甲酸酯和对羟基苯甲酸酯。 Aps 的生化表征揭示了其酯酶特性和更广泛的底物谱。不同侧链结构的对硝基苯酯(p-NB)在Aps作用下的降解速率不同,表现出对烷基侧链相对较长的底物的偏好。结构依赖性降解性与 Aps 和 p-NB 之间的结合能非常一致。分子对接和酶-底物相互作用阐明氢键、疏水相互作用和π-π堆积共同稳定酶-底物构象,促进底物水解。这些发现为芳香酯污染物的酶降解提供了新的见解,为混杂酶的进一步开发和修饰奠定了基础。
更新日期:2024-07-18
中文翻译:

混杂氨基肽酶水解多种芳香酯的底物表征
合成芳香酯广泛应用于农业、食品和化学工业,但由于其强疏水性和较差的生物利用度,已成为新兴的环境污染物。本研究试图通过在枯草芽孢杆菌中细胞外表达来自铜绿假单胞菌GF31 的混杂氨肽酶 (Aps) 来解决这个问题,实现了 13.7 U/mg 的令人印象深刻的酶活性。值得注意的是,我们首次证明了 Aps 介导的多种芳香酯的降解,包括但不限于拟除虫菊酯、邻苯二甲酸酯和对羟基苯甲酸酯。 Aps 的生化表征揭示了其酯酶特性和更广泛的底物谱。不同侧链结构的对硝基苯酯(p-NB)在Aps作用下的降解速率不同,表现出对烷基侧链相对较长的底物的偏好。结构依赖性降解性与 Aps 和 p-NB 之间的结合能非常一致。分子对接和酶-底物相互作用阐明氢键、疏水相互作用和π-π堆积共同稳定酶-底物构象,促进底物水解。这些发现为芳香酯污染物的酶降解提供了新的见解,为混杂酶的进一步开发和修饰奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号