当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integration of Melt Electrowritten Polymeric Scaffolds and Bioprinting for Epithelial Healing via Localized Periostin Delivery
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-07-18 , DOI: 10.1021/acsmacrolett.4c00240 Nileshkumar Dubey 1 , Maedeh Rahimnejad 2 , W Benton Swanson 3 , Jinping Xu 2 , Mylène de Ruijter 4, 5, 6 , Jos Malda 4, 5, 6 , Cristiane H Squarize 7 , Rogerio M Castilho 7 , Marco C Bottino 2, 8
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-07-18 , DOI: 10.1021/acsmacrolett.4c00240 Nileshkumar Dubey 1 , Maedeh Rahimnejad 2 , W Benton Swanson 3 , Jinping Xu 2 , Mylène de Ruijter 4, 5, 6 , Jos Malda 4, 5, 6 , Cristiane H Squarize 7 , Rogerio M Castilho 7 , Marco C Bottino 2, 8
Affiliation
|
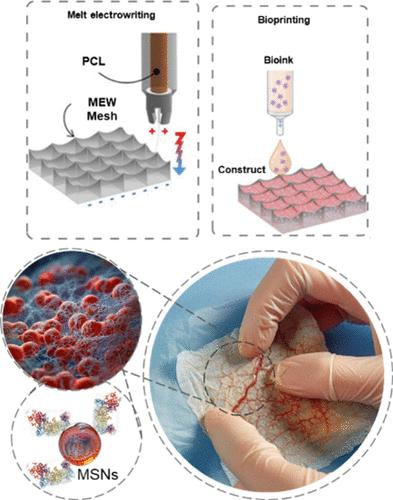
Management of skin injuries imposes a substantial financial burden on patients and hospitals, leading to diminished quality of life. Periostin (rhOSF), an extracellular matrix component, regulates cell function, including a proliferative healing phase, representing a key protein to promote wound healing. Despite its proven efficacy in vitro, there is a lack of scaffolds that facilitate the in situ delivery of rhOSF. In addition, there is a need for a scaffold to not only support cell growth, but also to resist the mechanical forces involved in wound healing. In this work, we synthesized rhOSF-loaded mesoporous nanoparticles (MSNs) and incorporated them into a cell-laden gelatin methacryloyl (GelMA) ink that was bioprinted into melt electrowritten poly(ε-caprolactone) (PCL) microfibrous (MF-PCL) meshes to develop mechanically competent constructs. Diffraction light scattering (DLS) analysis showed a narrow nanoparticle size distribution with an average size of 82.7 ± 13.2 nm. The rhOSF-loaded hydrogels showed a steady and controlled release of rhOSF over 16 days at a daily dose of ∼40 ng/mL. Compared with blank MSNs, the incorporation of rhOSF markedly augmented cell proliferation, underscoring its contribution to cellular performance. Our findings suggest a promising approach to address challenges such as prolonged healing, offering a potential solution for developing robust, biocompatible, and cell-laden grafts for burn wound healing applications.
中文翻译:

熔体电写聚合物支架和生物打印的整合通过局部骨膜素输送实现上皮愈合
皮肤损伤的处理给患者和医院带来了沉重的经济负担,导致生活质量下降。骨膜素 (rhOSF) 是一种细胞外基质成分,可调节细胞功能,包括增殖愈合阶段,是促进伤口愈合的关键蛋白质。尽管其在体外已被证明有效,但仍缺乏促进 rhOSF原位递送的支架。此外,支架不仅能支持细胞生长,还能抵抗伤口愈合所涉及的机械力。在这项工作中,我们合成了负载 rhOSF 的介孔纳米粒子 (MSN),并将其纳入载有细胞的明胶甲基丙烯酰 (GelMA) 墨水中,该墨水被生物打印到熔融电写聚(ε-己内酯) (PCL) 微纤维 (MF-PCL) 网格中开发机械性能良好的结构。衍射光散射 (DLS) 分析显示纳米颗粒尺寸分布较窄,平均尺寸为 82.7 ± 13.2 nm。负载 rhOSF 的水凝胶在 16 天内以~40 ng/mL 的每日剂量稳定且受控地释放 rhOSF。与空白 MSN 相比,rhOSF 的掺入显着增强了细胞增殖,强调了其对细胞性能的贡献。我们的研究结果提出了一种解决长期愈合等挑战的有前景的方法,为开发用于烧伤伤口愈合应用的坚固、生物相容性和充满细胞的移植物提供了潜在的解决方案。
更新日期:2024-07-18
中文翻译:

熔体电写聚合物支架和生物打印的整合通过局部骨膜素输送实现上皮愈合
皮肤损伤的处理给患者和医院带来了沉重的经济负担,导致生活质量下降。骨膜素 (rhOSF) 是一种细胞外基质成分,可调节细胞功能,包括增殖愈合阶段,是促进伤口愈合的关键蛋白质。尽管其在体外已被证明有效,但仍缺乏促进 rhOSF原位递送的支架。此外,支架不仅能支持细胞生长,还能抵抗伤口愈合所涉及的机械力。在这项工作中,我们合成了负载 rhOSF 的介孔纳米粒子 (MSN),并将其纳入载有细胞的明胶甲基丙烯酰 (GelMA) 墨水中,该墨水被生物打印到熔融电写聚(ε-己内酯) (PCL) 微纤维 (MF-PCL) 网格中开发机械性能良好的结构。衍射光散射 (DLS) 分析显示纳米颗粒尺寸分布较窄,平均尺寸为 82.7 ± 13.2 nm。负载 rhOSF 的水凝胶在 16 天内以~40 ng/mL 的每日剂量稳定且受控地释放 rhOSF。与空白 MSN 相比,rhOSF 的掺入显着增强了细胞增殖,强调了其对细胞性能的贡献。我们的研究结果提出了一种解决长期愈合等挑战的有前景的方法,为开发用于烧伤伤口愈合应用的坚固、生物相容性和充满细胞的移植物提供了潜在的解决方案。































 京公网安备 11010802027423号
京公网安备 11010802027423号