当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binary Cation Matrix Electrolyte and Its Effect on Solid Electrolyte Interphase Suppression and Evolution of Si Anode
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-18 , DOI: 10.1021/acsami.4c05194
Saida Cora 1 , John T Vaughey 2 , Niya Sa 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-18 , DOI: 10.1021/acsami.4c05194
Saida Cora 1 , John T Vaughey 2 , Niya Sa 1
Affiliation
|
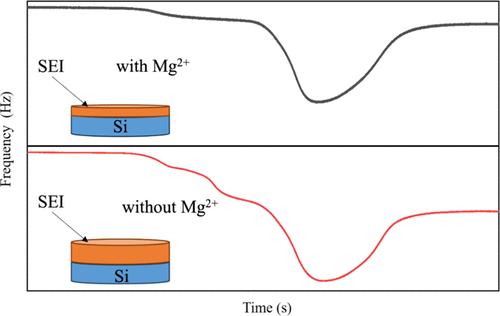
An unstable solid electrolyte interphase (SEI) has been recognized as one of the biggest challenges to commercializing silicon (Si) anodes for high-energy-density batteries. This work thoroughly investigates a binary cation matrix of Mg2++Li+ electrolyte and its role in SEI development, suppression, and evolution of a Si anode. Findings demonstrate that introducing Mg ions dramatically reduces the SEI growth before lithiation occurs, primarily due to the suppression of solvent reduction, particularly ethylene carbonate (EC) reduction. The Mg2+ alters the Li+ cation solvation environment as EC preferably participates in the oxophyllic Mg2+ solvation sheath, thereby altering the solvent reduction process, resulting in a distinct SEI formation mechanism. The initial SEI formation before lithiation is reduced by 70% in the electrolyte with the presence of Mg2+ cations. While the SEI continues to develop in the postlithiation, the inclusion of Mg ions results in an approximately 80% reduction in the postlithiation SEI growth. Continuous electrochemical cycling reveals that Mg2+ plays a crucial role in stabilizing the deep-lithiated Si phases, which effectively mitigates side reactions, resulting in controlled SEI growth and stable interphase while eliminating complex LixSiy formation. Mg ions promote the development of a notably more rigid and homogeneous SEI, characterized by a reduced dissipation (ΔD) in the Mg2++Li+ ion matrix compared to the solely Li+ system. This report reveals how the Mg2++Li+ ion matrix affects the SEI evolution, viscoelastic properties, and electrochemical behavior at the Si interface in real time, laying the groundwork for devising strategies to enhance the performance and longevity of Si-based next-generation battery systems.
中文翻译:

二元阳离子基质电解质及其对硅阳极固体电解质界面抑制和演化的影响
不稳定的固体电解质界面(SEI)已被认为是高能量密度电池硅(Si)阳极商业化的最大挑战之一。这项工作彻底研究了 Mg 2+ +Li +电解质的二元阳离子基质及其在 SEI 发展、抑制和硅阳极演变中的作用。研究结果表明,在锂化发生之前引入镁离子可显着降低 SEI 的生长,这主要是由于抑制了溶剂还原,特别是碳酸亚乙酯 (EC) 的还原。 Mg 2+改变了Li +阳离子溶剂化环境,因为EC优选参与亲氧Mg 2+溶剂化鞘,从而改变溶剂还原过程,导致独特的SEI形成机制。在存在 Mg 2+阳离子的电解质中,锂化前的初始 SEI 形成减少了 70%。虽然 SEI 在锂化后继续发展,但镁离子的加入导致锂化后 SEI 的生长减少了约 80%。连续电化学循环表明,Mg 2+在稳定深锂化Si相方面发挥着至关重要的作用,这有效地减轻了副反应,从而导致受控的SEI生长和稳定的界面,同时消除了复杂的Li x Si y形成。 Mg离子促进了显着更刚性和均匀的SEI的发展,其特征在于与单独的Li +体系相比,Mg 2+ +Li +离子基质中的耗散(Δ D )减少。 该报告揭示了 Mg 2+ +Li +离子基质如何实时影响 Si 界面的 SEI 演化、粘弹性性质和电化学行为,为制定提高硅基下一代材料性能和寿命的策略奠定了基础。发电电池系统。
更新日期:2024-07-18
中文翻译:

二元阳离子基质电解质及其对硅阳极固体电解质界面抑制和演化的影响
不稳定的固体电解质界面(SEI)已被认为是高能量密度电池硅(Si)阳极商业化的最大挑战之一。这项工作彻底研究了 Mg 2+ +Li +电解质的二元阳离子基质及其在 SEI 发展、抑制和硅阳极演变中的作用。研究结果表明,在锂化发生之前引入镁离子可显着降低 SEI 的生长,这主要是由于抑制了溶剂还原,特别是碳酸亚乙酯 (EC) 的还原。 Mg 2+改变了Li +阳离子溶剂化环境,因为EC优选参与亲氧Mg 2+溶剂化鞘,从而改变溶剂还原过程,导致独特的SEI形成机制。在存在 Mg 2+阳离子的电解质中,锂化前的初始 SEI 形成减少了 70%。虽然 SEI 在锂化后继续发展,但镁离子的加入导致锂化后 SEI 的生长减少了约 80%。连续电化学循环表明,Mg 2+在稳定深锂化Si相方面发挥着至关重要的作用,这有效地减轻了副反应,从而导致受控的SEI生长和稳定的界面,同时消除了复杂的Li x Si y形成。 Mg离子促进了显着更刚性和均匀的SEI的发展,其特征在于与单独的Li +体系相比,Mg 2+ +Li +离子基质中的耗散(Δ D )减少。 该报告揭示了 Mg 2+ +Li +离子基质如何实时影响 Si 界面的 SEI 演化、粘弹性性质和电化学行为,为制定提高硅基下一代材料性能和寿命的策略奠定了基础。发电电池系统。

































 京公网安备 11010802027423号
京公网安备 11010802027423号