Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of N-Alkyl-3-[2-oxoquinolin-1(2H)-yl]propanoic Acid Derivatives and Related Compounds: Cytotoxicity and EGFR Inhibition of Some Propanamide Derivatives
ACS Omega ( IF 3.7 ) Pub Date : 2024-07-16 , DOI: 10.1021/acsomega.4c03114 Samir M El Rayes 1 , Ibrahim A I Ali 1 , Walid Fathalla 2 , Mohamed A Ghanem 3 , Afaf H El-Sagheer 4 , Mohamed S Nafie 1, 5
ACS Omega ( IF 3.7 ) Pub Date : 2024-07-16 , DOI: 10.1021/acsomega.4c03114 Samir M El Rayes 1 , Ibrahim A I Ali 1 , Walid Fathalla 2 , Mohamed A Ghanem 3 , Afaf H El-Sagheer 4 , Mohamed S Nafie 1, 5
Affiliation
|
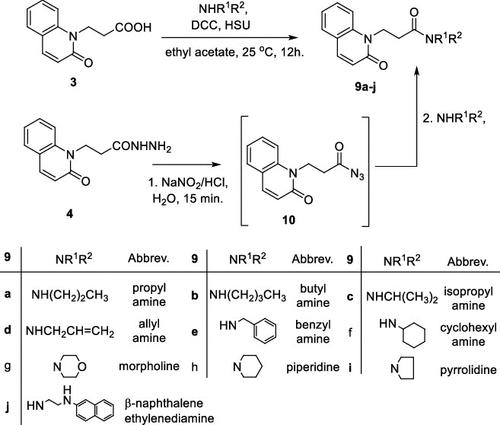
A series of 20 new structure-modified quinolin-2-one derivatives were prepared for biological evaluation. This was successfully achieved based on chemoselective reactions of heterocyclic amides with acrylic acid derivatives, which gave 3-[2-oxoquinolin-1-(2H)-yl] propanoic acid derivatives (N-substitution via a unique behavior). The ester was reacted with hydrazine to afford the corresponding hydrazide. Both the corresponding ester and hydrazide were used as building blocks to modify the quinolone structure and give N-hydroxyl propanamides, oxadiazoles, and thiosemicarbazides. The corresponding carboxylic acid and hydrazide were used to prepare several amides: N-alkyl-3-[2-oxoquinolin-1(2H)-yl]propanamides via azide and dicyclohexyl carbodiimide coupling methods. Among derivatives, compound 9e exhibited potent cytotoxicity against MCF-7 cells with an IC50 value of 1.32 μM compared to doxorubicin with an IC50 value of 1.21 μM. Additionally, it caused potent EGFR inhibition by 97% with an IC50 value of 16.89 nM compared to Erlotinib with an IC50 value of 29.8 nM. Finally, the binding mode of compound interactions toward EGFR was highlighted using a molecular docking study; compound 9e exhibited good binding affinity with a binding energy of −17.89 kcal/mol, and it formed H-bond interactions with Met 769 as the key amino acid of interaction. Accordingly, compound 9e may be developed as an EGFR-oriented chemotherapeutic antibreast cancer agent.
中文翻译:

N-烷基-3-[2-氧代喹啉-1(2H)-基]丙酸衍生物及相关化合物的合成:某些丙酰胺衍生物的细胞毒性和 EGFR 抑制
制备了一系列20种新型结构修饰的喹啉-2-酮衍生物用于生物学评价。这是基于杂环酰胺与丙烯酸衍生物的化学选择性反应成功实现的,得到3-[2-氧代喹啉-1-( 2H )-基]丙酸衍生物(通过独特行为进行N-取代)。该酯与肼反应得到相应的酰肼。相应的酯和酰肼都用作结构单元来修饰喹诺酮结构并得到N-羟基丙酰胺、恶二唑和氨基硫脲。相应的羧酸和酰肼用于通过叠氮化物和二环己基碳二亚胺偶联方法制备多种酰胺: N-烷基-3-[2-氧代喹啉-1( 2H )-基]丙酰胺。在衍生物中,化合物9e对MCF-7细胞表现出有效的细胞毒性,IC 50值为1.32 μM,而阿霉素的IC 50值为1.21 μM。此外,与 IC 50值为 29.8 nM 的厄洛替尼相比,它可有效抑制 97% 的 EGFR,IC 50值为 16.89 nM。最后,通过分子对接研究强调了化合物与 EGFR 相互作用的结合模式;化合物9e表现出良好的结合亲和力,结合能为-17.89 kcal/mol,并与作为相互作用关键氨基酸的Met 769形成氢键相互作用。因此,化合物9e可以开发为面向EGFR的化疗抗乳腺癌剂。
更新日期:2024-07-16
中文翻译:

N-烷基-3-[2-氧代喹啉-1(2H)-基]丙酸衍生物及相关化合物的合成:某些丙酰胺衍生物的细胞毒性和 EGFR 抑制
制备了一系列20种新型结构修饰的喹啉-2-酮衍生物用于生物学评价。这是基于杂环酰胺与丙烯酸衍生物的化学选择性反应成功实现的,得到3-[2-氧代喹啉-1-( 2H )-基]丙酸衍生物(通过独特行为进行N-取代)。该酯与肼反应得到相应的酰肼。相应的酯和酰肼都用作结构单元来修饰喹诺酮结构并得到N-羟基丙酰胺、恶二唑和氨基硫脲。相应的羧酸和酰肼用于通过叠氮化物和二环己基碳二亚胺偶联方法制备多种酰胺: N-烷基-3-[2-氧代喹啉-1( 2H )-基]丙酰胺。在衍生物中,化合物9e对MCF-7细胞表现出有效的细胞毒性,IC 50值为1.32 μM,而阿霉素的IC 50值为1.21 μM。此外,与 IC 50值为 29.8 nM 的厄洛替尼相比,它可有效抑制 97% 的 EGFR,IC 50值为 16.89 nM。最后,通过分子对接研究强调了化合物与 EGFR 相互作用的结合模式;化合物9e表现出良好的结合亲和力,结合能为-17.89 kcal/mol,并与作为相互作用关键氨基酸的Met 769形成氢键相互作用。因此,化合物9e可以开发为面向EGFR的化疗抗乳腺癌剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号