Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-19 , DOI: 10.1002/adsc.202400718 Cheng-Jie Wu 1 , Xu Jiang 1 , Jia-Yi Qiu 1 , Ming-Yang Wang 1 , Wei Li 1
|
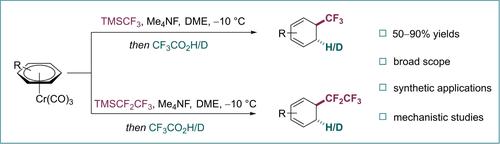
Dearomative trifluoromethylation of arenes provides an ideal method for constructing CF3-containing three-dimensional architectures which are of increasing interest in drug discovery. However, such transformation is rare and challenging because of the inherently low reactivity of arene π-systems and selectivity issues. Herein, we disclose a general dearomative 1,2-hydrotrifluoromethylation of (hetero)arenes utilizing activation by temporary π-complexation to chromium, thus affording convenient access to 1,3-cyclohexadienes bearing the privileged CF3 group. The versatility of this strategy further enables a dearomative 1,2-deuterotrifluoromethylation with high deuterated ratios. The related dearomative 1,2-(deutero)hydroperfluoroethylation reactions were conducted well for a range of chromium-bound (hetero)arenes. Finally, a reasonable mechanism was proposed based on the intermediate capture and control experiments.
中文翻译:

铬结合芳烃的去酰胺 1,2-(氘)氢三氟甲基化
芳烃的去体化三氟甲基化为构建含有 CF3 的三维结构提供了一种理想的方法,这在药物发现中越来越受到关注。然而,由于芳烃π系统固有的低反应性和选择性问题,这种转变很少见且具有挑战性。在此,我们公开了(异)芳烃的一般脱氢 1,2-氢三氟甲基化,利用临时 π 络合到铬的活化,从而提供了获得带有特权 CF3 基团的 1,3-环己二烯的便利。该策略的多功能性进一步实现了具有高氘代比的去氘化 1,2-氘三氟甲基化。相关的脱氢 1,2-(氘)氢全氟乙基化反应对一系列铬结合(异)芳烃进行良好。最后,在中间捕获和控制实验的基础上,提出了一种合理的机制。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号