当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interception and Synthetic Application of Diradical and Diene Forms of Dual-Nature Azabicyclic o-Quinodimethanes Generated by 6π-Azaelectrocyclization
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-07-18 , DOI: 10.1002/anie.202409613 Shankar Majji 1 , Daniel J Lee 2 , Supuni I N Hewa Inaththappulige 2 , Ayush Acharya 2 , Hemant P Yennawar 2 , Ramesh Giri 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-07-18 , DOI: 10.1002/anie.202409613 Shankar Majji 1 , Daniel J Lee 2 , Supuni I N Hewa Inaththappulige 2 , Ayush Acharya 2 , Hemant P Yennawar 2 , Ramesh Giri 3
Affiliation
|
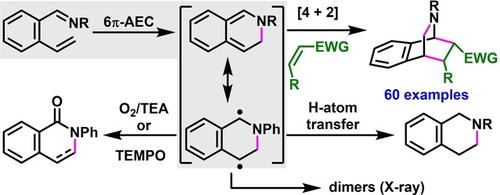
2-Alkenylarylimines have been shown to undergo 6π-azaelectrocyclization thermally to generate azabicyclic o-quinodimethanes. These o-quinodimethanes show dual nature as a cyclic diene and a benzylic diradical. The interception of the diradicaloid o-quinodimethanes by H-atom transfer enabled the synthesis of five tetrahydroisoquinoline alkaloids. The cyclic dienes were intercepted by [4+2] cycloaddition to generate bridgehead azabicycles.
中文翻译:

6π-氮杂电环化生成的双性质氮杂环邻喹二甲烷的双自由基和二烯形式的拦截和合成应用
2-烯基芳胺已被证明在热上发生 6π-氮杂电环化以生成氮杂环邻喹二甲烷。这些邻喹喹二甲烷表现出环状二烯和苄基二自由基的双重性质。通过 H 原子转移拦截双自由基类 o-喹啉二甲烷,能够合成五种四氢异喹啉生物碱。环状二烯被 [4+2] 环加成反应拦截,生成桥头扎扎自行车。
更新日期:2024-07-18
中文翻译:

6π-氮杂电环化生成的双性质氮杂环邻喹二甲烷的双自由基和二烯形式的拦截和合成应用
2-烯基芳胺已被证明在热上发生 6π-氮杂电环化以生成氮杂环邻喹二甲烷。这些邻喹喹二甲烷表现出环状二烯和苄基二自由基的双重性质。通过 H 原子转移拦截双自由基类 o-喹啉二甲烷,能够合成五种四氢异喹啉生物碱。环状二烯被 [4+2] 环加成反应拦截,生成桥头扎扎自行车。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号