Basic Research in Cardiology ( IF 7.5 ) Pub Date : 2024-07-17 , DOI: 10.1007/s00395-024-01068-8 Tamás G Gergely 1, 2, 3 , Zsófia D Drobni 4 , Nabil V Sayour 1, 2, 3 , Péter Ferdinandy 1, 5 , Zoltán V Varga 1, 2, 3
|
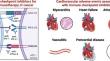
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy by unleashing the power of the immune system against malignant cells. However, their use is associated with a spectrum of adverse effects, including cardiovascular complications, which can pose significant clinical challenges. Several mechanisms contribute to cardiovascular toxicity associated with ICIs. First, the dysregulation of immune checkpoints, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1) and its ligand (PD-L1), and molecular mimicry with cardiac autoantigens, leads to immune-related adverse events, including myocarditis and vasculitis. These events result from the aberrant activation of T cells against self-antigens within the myocardium or vascular endothelium. Second, the disruption of immune homeostasis by ICIs can lead to autoimmune-mediated inflammation of cardiac tissues, manifesting as cardiac dysfunction and heart failure, arrhythmias, or pericarditis. Furthermore, the upregulation of inflammatory cytokines, particularly tumor necrosis factor-alpha, interferon-γ, interleukin-1β, interleukin-6, and interleukin-17 contributes to cardiac and endothelial dysfunction, plaque destabilization, and thrombosis, exacerbating cardiovascular risk on the long term. Understanding the intricate mechanisms of cardiovascular side effects induced by ICIs is crucial for optimizing patient care and to ensure the safe and effective integration of immunotherapy into a broader range of cancer treatment protocols. The clinical implications of these mechanisms underscore the importance of vigilant monitoring and early detection of cardiovascular toxicity in patients receiving ICIs. Future use of these key pathological mediators as biomarkers may aid in prompt diagnosis of cardiotoxicity and will allow timely interventions.
中文翻译:

免疫检查点抑制剂心血管毒性的分子指纹
免疫检查点抑制剂(ICIs)通过释放免疫系统对抗恶性细胞的力量,彻底改变了癌症治疗。然而,它们的使用会带来一系列不良反应,包括心血管并发症,这可能会带来重大的临床挑战。有多种机制会导致 ICI 相关的心血管毒性。首先,免疫检查点的失调,例如细胞毒性T淋巴细胞相关蛋白4(CTLA-4)和程序性细胞死亡蛋白1(PD-1)及其配体(PD-L1),以及心脏自身抗原的分子模拟,导致免疫相关的不良事件,包括心肌炎和血管炎。这些事件是由于心肌或血管内皮内 T 细胞针对自身抗原的异常激活所致。其次,ICIs 破坏免疫稳态可导致自身免疫介导的心脏组织炎症,表现为心功能障碍和心力衰竭、心律失常或心包炎。此外,炎症细胞因子的上调,特别是肿瘤坏死因子-α、干扰素-γ、白细胞介素-1β、白细胞介素-6和白细胞介素-17,会导致心脏和内皮功能障碍、斑块不稳定和血栓形成,长期加剧心血管风险。学期。了解 ICI 引起的心血管副作用的复杂机制对于优化患者护理并确保将免疫疗法安全有效地整合到更广泛的癌症治疗方案中至关重要。这些机制的临床意义强调了对接受 ICI 的患者进行警惕监测和早期检测心血管毒性的重要性。 未来使用这些关键病理介质作为生物标志物可能有助于及时诊断心脏毒性,并可以及时进行干预。






























 京公网安备 11010802027423号
京公网安备 11010802027423号