当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cartilage extracellular matrix polymers: hierarchical structure, osmotic properties, and function
Soft Matter ( IF 2.9 ) Pub Date : 2024-07-19 , DOI: 10.1039/d4sm00617h Ferenc Horkay 1 , Peter J Basser 1 , Erik Geissler 2
Soft Matter ( IF 2.9 ) Pub Date : 2024-07-19 , DOI: 10.1039/d4sm00617h Ferenc Horkay 1 , Peter J Basser 1 , Erik Geissler 2
Affiliation
|
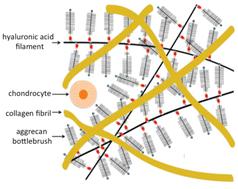
Proteoglycans are hierarchically organized structures that play an important role in the hydration and the compression resistance of cartilage matrix. In this study, the static and dynamic properties relevant to the biomechanical function of cartilage are determined at different levels of the hierarchical structure, using complementary osmotic pressure, neutron scattering (SANS) and light scattering (DLS) measurements. In cartilage proteoglycans (PGs), two levels of bottlebrush structures can be distinguished: the aggrecan monomer, which consists of a core protein to which are tethered charged glycosaminoglycan (GAG) chains, and complexes formed of the aggrecan monomers attached around a linear hyaluronic acid backbone. The principal component of GAG, chondroitin sulfate (CS), is used as a baseline in this comparison. The osmotic modulus, measured as a function of the proteoglycan concentration, follows the order CS < aggrecan < aggrecan–HA complex. This order underlines the benefit of the increasing complexity at each level of the molecular architecture. The hierarchical bottlebrush configuration, which prevents interpenetration among the bristles of the aggrecan monomers, enhances both the mechanical properties and the osmotic resistance. The osmotic pressure of the collagen solution is notably smaller than in the proteoglycan systems. This is consistent with its known primary role to provide tensile strength to the cartilage and to confine the aggrecan–HA complexes, as opposed to load bearing. The collective diffusion coefficient D governs the rate of recovery of biological tissue after compressive load. In CS solutions the diffusion process is fast, D ≈ 3 × 10−6 cm2 s−1 at concentrations comparable with that of the GAG chains inside the aggrecan molecule. In CS solutions D is a weakly decreasing function of calcium ion concentration, while in aggrecan and its complexes with HA, the relaxation rate is insensitive to the presence of calcium.
中文翻译:

软骨细胞外基质聚合物:层次结构、渗透特性和功能
蛋白聚糖是分层组织的结构,在软骨基质的水合作用和抗压性中发挥重要作用。在这项研究中,利用互补渗透压、中子散射 (SANS) 和光散射 (DLS) 测量,在分层结构的不同级别确定与软骨生物力学功能相关的静态和动态特性。在软骨蛋白聚糖 (PG) 中,可以区分两个层次的洗瓶刷结构:聚集蛋白聚糖单体(由束缚有带电糖胺聚糖 (GAG) 链的核心蛋白组成)和由附着在线性透明质酸周围的聚集蛋白聚糖单体形成的复合物骨干。 GAG 的主要成分硫酸软骨素 (CS) 被用作本次比较的基线。渗透模量作为蛋白聚糖浓度的函数测量,遵循 CS < 聚集蛋白聚糖 < 聚集蛋白聚糖-HA 复合物的顺序。这一顺序强调了分子结构各个层面复杂性不断增加的好处。分层的洗瓶刷结构可以防止聚集蛋白聚糖单体的刷毛之间的相互渗透,从而增强了机械性能和渗透阻力。胶原蛋白溶液的渗透压明显小于蛋白聚糖系统中的渗透压。这与其已知的主要作用是一致的,即为软骨提供拉伸强度并限制聚集蛋白聚糖-HA复合物,而不是承载。集体扩散系数D控制生物组织在压缩负载后的恢复率。 在CS溶液中,扩散过程很快, D ≈ 3 × 10 -6 cm 2 s -1 ,浓度与聚集蛋白聚糖分子内的GAG链相当。在CS溶液中, D是钙离子浓度的弱递减函数,而在聚集蛋白聚糖及其与HA的复合物中,弛豫率对钙的存在不敏感。
更新日期:2024-07-19
中文翻译:

软骨细胞外基质聚合物:层次结构、渗透特性和功能
蛋白聚糖是分层组织的结构,在软骨基质的水合作用和抗压性中发挥重要作用。在这项研究中,利用互补渗透压、中子散射 (SANS) 和光散射 (DLS) 测量,在分层结构的不同级别确定与软骨生物力学功能相关的静态和动态特性。在软骨蛋白聚糖 (PG) 中,可以区分两个层次的洗瓶刷结构:聚集蛋白聚糖单体(由束缚有带电糖胺聚糖 (GAG) 链的核心蛋白组成)和由附着在线性透明质酸周围的聚集蛋白聚糖单体形成的复合物骨干。 GAG 的主要成分硫酸软骨素 (CS) 被用作本次比较的基线。渗透模量作为蛋白聚糖浓度的函数测量,遵循 CS < 聚集蛋白聚糖 < 聚集蛋白聚糖-HA 复合物的顺序。这一顺序强调了分子结构各个层面复杂性不断增加的好处。分层的洗瓶刷结构可以防止聚集蛋白聚糖单体的刷毛之间的相互渗透,从而增强了机械性能和渗透阻力。胶原蛋白溶液的渗透压明显小于蛋白聚糖系统中的渗透压。这与其已知的主要作用是一致的,即为软骨提供拉伸强度并限制聚集蛋白聚糖-HA复合物,而不是承载。集体扩散系数D控制生物组织在压缩负载后的恢复率。 在CS溶液中,扩散过程很快, D ≈ 3 × 10 -6 cm 2 s -1 ,浓度与聚集蛋白聚糖分子内的GAG链相当。在CS溶液中, D是钙离子浓度的弱递减函数,而在聚集蛋白聚糖及其与HA的复合物中,弛豫率对钙的存在不敏感。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号