当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical study on the carbon nanomaterial-supported Pt complex electrocatalysts for efficient and selective chlorine evolution reaction
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-07-17 , DOI: 10.1002/jcc.27466 Jewel Hossen 1, 2 , Naoki Nakatani 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-07-17 , DOI: 10.1002/jcc.27466 Jewel Hossen 1, 2 , Naoki Nakatani 1
Affiliation
|
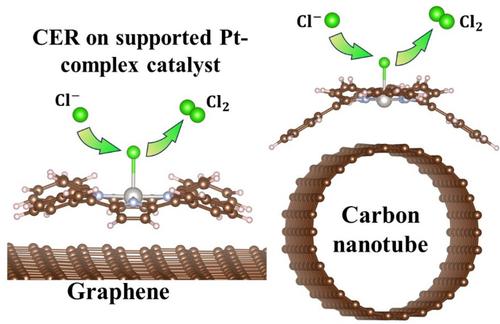
Chlorine is an important chemical which has long been produced in chlor-alkali process using dimensionally stable anodes (DSA). However, some serious drawbacks of DSA inspire the development of alternative anodes for chlorine evolution reaction (CER). In this study, we focused on the graphene- and carbon nanotube-supported platinum tetra-phenyl porphyrins as electrocatalysts for CER, which have been theoretically investigated based on density functional theory. Our results reveal that the supported substrates possess potential CER electrocatalytic activity with very low thermodynamic overpotentials (0.012–0.028 V) via Cl* pathway instead of ClO*. The electronic structures analyses showed that electron transfer from the support to the adsorbed chlorine via the Pt center leads to strong Pt–Cl interactions. Furthermore, the supported electrocatalysts exhibited excellent selectivity toward CER because of high overpotentials and reaction barriers of oxygen evolution process. Therefore, our results may pave the way for designing CER electrocatalyst utilizing emerging carbon nanomaterials.
中文翻译:

碳纳米材料负载的Pt络合物电催化剂在高效选择性析氯反应中的理论研究
氯是一种重要的化学品,长期以来一直使用尺寸稳定阳极 (DSA) 在氯碱工艺中生产。然而,DSA 的一些严重缺点激发了用于析氯反应 (CER) 的替代阳极的开发。在这项研究中,我们专注于石墨烯和碳纳米管负载的铂四苯基卟啉作为 CER 的电催化剂,这些卟啉已基于密度泛函理论进行了理论研究。我们的结果表明,负载的底物通过 Cl* 途径而不是 ClO* 具有潜在的 CER 电催化活性和非常低的热力学过电位 (0.012–0.028 V)。电子结构分析表明,电子通过 Pt 中心从载体转移到吸附的氯会导致强烈的 Pt-Cl 相互作用。此外,由于析氧过程的高过电位和反应势垒,负载型电催化剂对 CER 表现出优异的选择性。因此,我们的结果可能为利用新兴碳纳米材料设计 CER 电催化剂铺平道路。
更新日期:2024-07-17
中文翻译:

碳纳米材料负载的Pt络合物电催化剂在高效选择性析氯反应中的理论研究
氯是一种重要的化学品,长期以来一直使用尺寸稳定阳极 (DSA) 在氯碱工艺中生产。然而,DSA 的一些严重缺点激发了用于析氯反应 (CER) 的替代阳极的开发。在这项研究中,我们专注于石墨烯和碳纳米管负载的铂四苯基卟啉作为 CER 的电催化剂,这些卟啉已基于密度泛函理论进行了理论研究。我们的结果表明,负载的底物通过 Cl* 途径而不是 ClO* 具有潜在的 CER 电催化活性和非常低的热力学过电位 (0.012–0.028 V)。电子结构分析表明,电子通过 Pt 中心从载体转移到吸附的氯会导致强烈的 Pt-Cl 相互作用。此外,由于析氧过程的高过电位和反应势垒,负载型电催化剂对 CER 表现出优异的选择性。因此,我们的结果可能为利用新兴碳纳米材料设计 CER 电催化剂铺平道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号