当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Diastereo-, Enantio-, and (Z)-Selective Aminoallylation of Ketones through Reductive Couplings of Azatrienes for the Synthesis of Allylic 1,2-Amino Tertiary Alcohols
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-16 , DOI: 10.1021/jacs.4c05637 Jiaqi Zhu 1 , Faraan Rahim 1 , Pengfei Zhou 1 , Annie Zhang 1 , Steven J Malcolmson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-16 , DOI: 10.1021/jacs.4c05637 Jiaqi Zhu 1 , Faraan Rahim 1 , Pengfei Zhou 1 , Annie Zhang 1 , Steven J Malcolmson 1
Affiliation
|
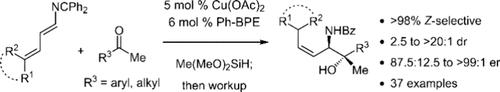
We introduce a method for the (Z)-selective aminoallylation of a range of ketones to prepare allylic 1,2-amino tertiary alcohols with excellent diastereo- and enantioselectivity. Copper-catalyzed reductive couplings of 2-azatrienes with aryl/alkyl and dialkyl ketones proceed with Ph-BPE as the supporting ligand, generating anti-amino alcohols with >98% (Z)-selectivity under mild conditions. The utility of the products is highlighted through several transformations, including those that leverage the (Z)-allylic amine moiety for diastereoselective reactions of the alkene. Calculations illustrate Curtin–Hammett control in the product formation over other possible isomers and the origin of (Z)-selectivity.
中文翻译:

铜催化酮通过氮杂三烯的还原偶联进行非对映、对映和 (Z)-选择性氨基烯丙基化,用于合成烯丙基 1,2-氨基叔醇
我们介绍了一种对一系列酮进行 ( Z )-选择性氨基烯丙基化来制备具有优异的非对映选择性和对映选择性的烯丙基 1,2-氨基叔醇的方法。以 Ph-BPE 作为支持配体,铜催化 2-氮杂三烯与芳基/烷基和二烷基酮进行还原偶联,在温和条件下生成选择性 >98% ( Z ) 的反氨基醇。该产品的实用性通过多种转化得以凸显,包括利用 ( Z )-烯丙基胺部分进行烯烃的非对映选择性反应。计算表明科廷-哈米特对产物形成的控制优于其他可能的异构体以及 ( Z )-选择性的起源。
更新日期:2024-07-16
中文翻译:

铜催化酮通过氮杂三烯的还原偶联进行非对映、对映和 (Z)-选择性氨基烯丙基化,用于合成烯丙基 1,2-氨基叔醇
我们介绍了一种对一系列酮进行 ( Z )-选择性氨基烯丙基化来制备具有优异的非对映选择性和对映选择性的烯丙基 1,2-氨基叔醇的方法。以 Ph-BPE 作为支持配体,铜催化 2-氮杂三烯与芳基/烷基和二烷基酮进行还原偶联,在温和条件下生成选择性 >98% ( Z ) 的反氨基醇。该产品的实用性通过多种转化得以凸显,包括利用 ( Z )-烯丙基胺部分进行烯烃的非对映选择性反应。计算表明科廷-哈米特对产物形成的控制优于其他可能的异构体以及 ( Z )-选择性的起源。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号