当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Construction of Chiral Polysubstituted 3-Azabicyclo[3.1.1]heptanes by Copper-Catalyzed Stereoselective Formal [4π+2σ] Cycloaddition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-16 , DOI: 10.1021/jacs.4c06436 Xunhua Wang 1 , Rongkai Gao 1 , Xiaoxun Li 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-16 , DOI: 10.1021/jacs.4c06436 Xunhua Wang 1 , Rongkai Gao 1 , Xiaoxun Li 1, 2
Affiliation
|
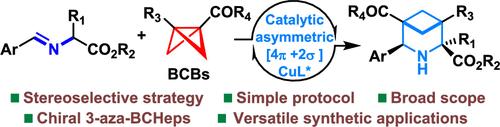
The direct construction of bioisosteric compounds enriched in Csp3 content represents an attractive and dependable approach to imbuing biologically active molecules with enhanced three-dimensional characteristics, finding wide utility across the synthetic and medicinal chemistry community. Despite recent advancements in the synthesis of (aza)-bicyclo[3.1.1]heptanes (BCHeps and aza-BCHeps), which serve as meta-substituted (aza)-arene bioisosteres, the enantioselective assembly of chiral 3-aza-BCHeps remains a coveted goal yet to be achieved. Here, we disclose an unprecedented copper-catalyzed asymmetric formal [4π+2σ] cycloaddition of bicyclo[1.1.0]butanes (BCBs) and azomethine ylides, furnishing a diverse array of enantioenriched 3-aza-BCHeps with exceptional levels of diastereo- and enantioselectivites (51 examples, all >20:1 dr, mostly 97–99% ee). Both mono- and disubstituted BCBs are well compatible with this protocol, offering an enticing route for the efficient synthesis of challenging tetrasubstituted bicyclic products bearing two quaternary centers. The synthetic significance of this methodology is further demonstrated by the successful preparation of several piperidine drug analogues.
中文翻译:

铜催化立体选择性形式[4π+2σ]环加成催化不对称构建手性多取代3-氮杂双环[3.1.1]庚烷
直接构建富含 C sp3含量的生物电子等排化合物代表了一种有吸引力且可靠的方法,可以为生物活性分子赋予增强的三维特性,在合成和药物化学领域具有广泛的用途。尽管最近在(氮杂)-双环[3.1.1]庚烷(BCHeps和氮杂-BCHeps)(作为间位取代的(氮杂)-芳烃生物等排体)的合成方面取得了进展,但手性3-氮杂-BCHeps的对映选择性组装仍然存在一个令人垂涎的目标尚未实现。在这里,我们公开了一种前所未有的铜催化双环[1.1.0]丁烷(BCB)和甲亚碱叶立德的不对称形式[4π+2σ]环加成反应,提供了多种对映体富集的3-aza-BCHeps,具有异常水平的非对映和对映选择性(51 个例子,全部 >20:1 dr,大部分为 97–99% ee)。单取代和双取代的 BCB 都与该方案良好兼容,为有效合成具有两个四元中心的具有挑战性的四取代双环产物提供了一条诱人的途径。几种哌啶药物类似物的成功制备进一步证明了该方法的合成意义。
更新日期:2024-07-16
中文翻译:

铜催化立体选择性形式[4π+2σ]环加成催化不对称构建手性多取代3-氮杂双环[3.1.1]庚烷
直接构建富含 C sp3含量的生物电子等排化合物代表了一种有吸引力且可靠的方法,可以为生物活性分子赋予增强的三维特性,在合成和药物化学领域具有广泛的用途。尽管最近在(氮杂)-双环[3.1.1]庚烷(BCHeps和氮杂-BCHeps)(作为间位取代的(氮杂)-芳烃生物等排体)的合成方面取得了进展,但手性3-氮杂-BCHeps的对映选择性组装仍然存在一个令人垂涎的目标尚未实现。在这里,我们公开了一种前所未有的铜催化双环[1.1.0]丁烷(BCB)和甲亚碱叶立德的不对称形式[4π+2σ]环加成反应,提供了多种对映体富集的3-aza-BCHeps,具有异常水平的非对映和对映选择性(51 个例子,全部 >20:1 dr,大部分为 97–99% ee)。单取代和双取代的 BCB 都与该方案良好兼容,为有效合成具有两个四元中心的具有挑战性的四取代双环产物提供了一条诱人的途径。几种哌啶药物类似物的成功制备进一步证明了该方法的合成意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号