当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Reduction of Esters over Zirconia-Supported Metal Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-17 , DOI: 10.1021/acscatal.4c01025 Javier E. Chavarrio 1 , Kyle Kirkendall-Jones 1 , Raka G. Dastidar 1 , George W. Huber 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-17 , DOI: 10.1021/acscatal.4c01025 Javier E. Chavarrio 1 , Kyle Kirkendall-Jones 1 , Raka G. Dastidar 1 , George W. Huber 1
Affiliation
|
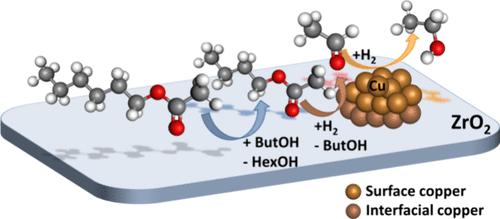
Esters are often produced as unwanted byproducts during the catalytic upgrading of ethanol to diesel fuel precursors through Guerbet coupling. Removal of esters from the product stream is important to prevent the loss of downstream catalyst activity from ester-derived carboxylic acids. In this work, we studied ester hydrogenolysis to the parent alcohols as a viable route for enhanced diesel fuel production. Specifically, we investigated the reduction of hexyl acetate in butanol over ZrO2-supported Ni, Co, Cu, Rh, Pd, and Pt catalysts, where Cu/ZrO2 was the most selective catalyst for the hydrogenolysis of hexyl acetate into hexanol and ethanol. Thermodynamic analysis reveals that a 90% alcohol yield can be obtained at 200 °C, 30 bar, and a relatively high H2:hexyl acetate molar ratio of 480:1. Experimentally, an alcohol yield of 88% yield was obtained with a 10 wt % Cu/ZrO2 catalyst at these conditions with a residence time of 5.4 h kgcat kmolgas–1. Catalytic tests on the support revealed that ZrO2 catalyzes the transesterification reaction between hexyl acetate and butanol. However, only the Cu sites can catalyze the hydrogenolysis of the esters into the final alcohols. We developed a kinetic model for our experimental results, which shows that the transesterification and hydrogenolysis reactions run at two different timescales, the former being 10 times faster than the latter. Data regression has been used to develop a model to predict the mole fraction distribution of ester hydrogenolysis products over a wide range of contact times. Cu/ZrO2 loses half its catalytic activity after 80 h of time on stream. Modeling of deactivation data reveals that the ZrO2 support conserves a residual activity due to external active sites, while active sites over the Cu surface deactivate at different rates. The catalytic conversion of esters into their parent alcohols is relevant to the production of surrogate liquid fuels since alcohols can be bimolecularly dehydrated to produce a blend of ethers with diesel fuel-like properties.
中文翻译:

氧化锆负载金属催化剂催化还原酯
在通过格尔伯特偶联将乙醇催化升级为柴油燃料前体的过程中,酯通常作为不需要的副产物产生。从产物流中去除酯对于防止酯衍生的羧酸损失下游催化剂活性非常重要。在这项工作中,我们研究了酯氢解为母体醇作为提高柴油产量的可行途径。具体来说,我们研究了 ZrO 2 负载的 Ni、Co、Cu、Rh、Pd 和 Pt 催化剂上丁醇中乙酸己酯的还原,其中 Cu/ZrO 2 最有效用于乙酸己酯氢解成己醇和乙醇的选择性催化剂。热力学分析表明,在200℃、30bar和相对较高的H 2 :乙酸己酯摩尔比(480:1)下可以获得90%的醇产率。实验上,在这些条件下,使用 10 wt% Cu/ZrO 2 催化剂,停留时间为 5.4 h kg cat kmol gas 。载体催化测试表明,ZrO 2 能够催化乙酸己酯与丁醇的酯交换反应。然而,只有铜位点可以催化酯氢解成最终的醇。我们为实验结果开发了一个动力学模型,该模型表明酯交换和氢解反应在两个不同的时间尺度上运行,前者比后者快 10 倍。数据回归已用于开发模型来预测酯氢解产物在较宽的接触时间范围内的摩尔分数分布。 Cu/ZrO 2 在运行 80 小时后失去一半的催化活性。 失活数据建模表明,ZrO 2 载体由于外部活性位点而保留了残余活性,而 Cu 表面上的活性位点以不同的速率失活。酯催化转化为其母体醇与替代液体燃料的生产相关,因为醇可以双分子脱水以产生具有柴油燃料性质的醚混合物。
更新日期:2024-07-17
中文翻译:

氧化锆负载金属催化剂催化还原酯
在通过格尔伯特偶联将乙醇催化升级为柴油燃料前体的过程中,酯通常作为不需要的副产物产生。从产物流中去除酯对于防止酯衍生的羧酸损失下游催化剂活性非常重要。在这项工作中,我们研究了酯氢解为母体醇作为提高柴油产量的可行途径。具体来说,我们研究了 ZrO 2 负载的 Ni、Co、Cu、Rh、Pd 和 Pt 催化剂上丁醇中乙酸己酯的还原,其中 Cu/ZrO 2 最有效用于乙酸己酯氢解成己醇和乙醇的选择性催化剂。热力学分析表明,在200℃、30bar和相对较高的H 2 :乙酸己酯摩尔比(480:1)下可以获得90%的醇产率。实验上,在这些条件下,使用 10 wt% Cu/ZrO 2 催化剂,停留时间为 5.4 h kg cat kmol gas 。载体催化测试表明,ZrO 2 能够催化乙酸己酯与丁醇的酯交换反应。然而,只有铜位点可以催化酯氢解成最终的醇。我们为实验结果开发了一个动力学模型,该模型表明酯交换和氢解反应在两个不同的时间尺度上运行,前者比后者快 10 倍。数据回归已用于开发模型来预测酯氢解产物在较宽的接触时间范围内的摩尔分数分布。 Cu/ZrO 2 在运行 80 小时后失去一半的催化活性。 失活数据建模表明,ZrO 2 载体由于外部活性位点而保留了残余活性,而 Cu 表面上的活性位点以不同的速率失活。酯催化转化为其母体醇与替代液体燃料的生产相关,因为醇可以双分子脱水以产生具有柴油燃料性质的醚混合物。












































 京公网安备 11010802027423号
京公网安备 11010802027423号