当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Surfaces and Confinement on Formic Acid Dehydrogenation Catalyzed by an Immobilized Ru–H Complex: Insights from Molecular Simulation and Neutron Scattering
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-13 , DOI: 10.1021/acscatal.4c02626 Hoang-Huy Nguyen 1 , Marc Högler 2 , Nadine Schnabel 1 , Niels Hansen 2 , Thomas Sottmann 1 , Deven P. Estes 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-13 , DOI: 10.1021/acscatal.4c02626 Hoang-Huy Nguyen 1 , Marc Högler 2 , Nadine Schnabel 1 , Niels Hansen 2 , Thomas Sottmann 1 , Deven P. Estes 1
Affiliation
|
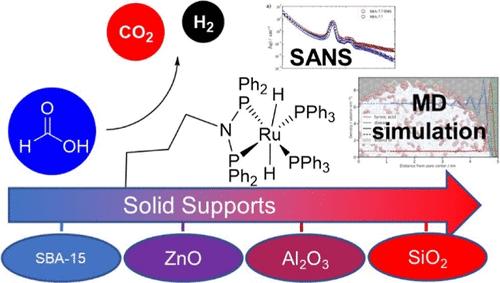
Formic acid is a potential liquid hydrogen carrier (LHC) for storage and transport of hydrogen, which requires the rapid and efficient recovery of hydrogen from formic acid (through catalysis). Various homogeneous complexes fulfill all of the desired criteria for a formic acid dehydrogenation catalyst. Immobilization of such complexes on solid supports is a common strategy for their application industrially in flow reactors. However, the properties of the support can change the reactivity of the catalyst, often reducing the catalytic activity in ways that are difficult to predict. Here we examine the effect of supports on the dehydrogenation of formic acid using H2Ru(PPh3)2(PPh2)2N–C3H6–Si(OEt)3 (1) through a combination of kinetic measurements, molecular dynamics (MD) simulations, and small-angle neutron scattering (SANS). Immobilization of 1 on all supports decreases the rate of formic acid conversion in the order SiO2 > Al2O3 > ZnO > SBA-TMS > SBA-15. Combination of MD simulations and SANS shows that high affinity of formic acid for the surfaces and in particular when confined in the pores results in a high local FA concentration, which inhibits the catalytic rate. Collapse of the Ru complex onto the surface/pore wall may also contribute to this overall inhibition.
中文翻译:

表面和约束对固定化 Ru-H 配合物催化甲酸脱氢的影响:来自分子模拟和中子散射的见解
甲酸是一种潜在的用于储存和运输氢气的液态氢载体(LHC),这需要从甲酸中快速有效地回收氢气(通过催化)。各种均相配合物满足甲酸脱氢催化剂的所有所需标准。将此类复合物固定在固体载体上是其在流动反应器中工业应用的常见策略。然而,载体的性质可以改变催化剂的反应性,通常以难以预测的方式降低催化活性。在这里,我们使用 H 2 Ru(PPh 3 ) 2 (PPh 2 ) 检查载体对甲酸脱氢的影响 2 N–C 3 H 6 –Si(OEt) 3 (1) 通过结合动力学测量、分子动力学 ( MD)模拟和小角中子散射(SANS)。 1 在所有载体上的固定化会降低甲酸转化率,顺序为 SiO 2 > Al 2 O 3 > ZnO > SBA-TMS > SBA- 15. MD 模拟和 SANS 的结合表明,甲酸对表面的高亲和力,特别是当限制在孔中时,会导致局部 FA 浓度较高,从而抑制催化速率。 Ru 络合物在表面/孔壁上的塌陷也可能有助于这种总体抑制。
更新日期:2024-07-17
中文翻译:

表面和约束对固定化 Ru-H 配合物催化甲酸脱氢的影响:来自分子模拟和中子散射的见解
甲酸是一种潜在的用于储存和运输氢气的液态氢载体(LHC),这需要从甲酸中快速有效地回收氢气(通过催化)。各种均相配合物满足甲酸脱氢催化剂的所有所需标准。将此类复合物固定在固体载体上是其在流动反应器中工业应用的常见策略。然而,载体的性质可以改变催化剂的反应性,通常以难以预测的方式降低催化活性。在这里,我们使用 H 2 Ru(PPh 3 ) 2 (PPh 2 ) 检查载体对甲酸脱氢的影响 2 N–C 3 H 6 –Si(OEt) 3 (1) 通过结合动力学测量、分子动力学 ( MD)模拟和小角中子散射(SANS)。 1 在所有载体上的固定化会降低甲酸转化率,顺序为 SiO 2 > Al 2 O 3 > ZnO > SBA-TMS > SBA- 15. MD 模拟和 SANS 的结合表明,甲酸对表面的高亲和力,特别是当限制在孔中时,会导致局部 FA 浓度较高,从而抑制催化速率。 Ru 络合物在表面/孔壁上的塌陷也可能有助于这种总体抑制。












































 京公网安备 11010802027423号
京公网安备 11010802027423号