当前位置:
X-MOL 学术
›
Nano Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weakly solvating ester electrolyte for high voltage sodium-ion batteries
Nano Energy ( IF 16.8 ) Pub Date : 2024-07-05 , DOI: 10.1016/j.nanoen.2024.109969 Rishivandhiga Jayakumar , Travis P. Pollard , Oleg Borodin , Vadim Shipitsyn , Chanmonirath (Michael) Chak , Glenn Pastel , Allen Zheng , Michel Johnson , Fuead Hasan , Christopher M. Bejger , Marshall A. Schroeder , Steve G. Greenbaum , Wenhua Zuo , Lin Ma
Nano Energy ( IF 16.8 ) Pub Date : 2024-07-05 , DOI: 10.1016/j.nanoen.2024.109969 Rishivandhiga Jayakumar , Travis P. Pollard , Oleg Borodin , Vadim Shipitsyn , Chanmonirath (Michael) Chak , Glenn Pastel , Allen Zheng , Michel Johnson , Fuead Hasan , Christopher M. Bejger , Marshall A. Schroeder , Steve G. Greenbaum , Wenhua Zuo , Lin Ma
|
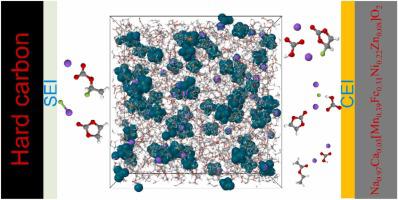
Ethyl acetate (EA) was identified as a promising electrolyte solvent for sodium-ion batteries (SIBs), exhibiting low viscosity, cost-effectiveness, and low toxicity. Despite a significant portion of aggregation being linked to the weak solvation of Na+ /EA as revealed by molecular dynamics (MD) simulations, pulsed-field gradient nuclear magnetic resonance (pfg-NMR) analysis identified a noteworthy Na+ diffusion coefficient of 3.95×10−10 m2 s−1 at 25°C in the presence of 1 m NaPF6 salt. Employing fluoroethylene carbonate (FEC) as a film-forming additive to create electrode-electrolyte interphase, this electrolyte surprisingly made ∼210 mAh Na0.97 Ca0.03 [Mn0.39 Fe0.31 Ni0.22 Zn0.08 ]O2 (NCMFNZO)/hard carbon (HC) pouch cells achieve a lengthy cycling lifetime of 250 cycles with ∼80 % capacity retention, cycled up to 4.0 V at 40°C. X-ray photoelectron spectroscopy (XPS) revealed increasing interphasial organic species over cycling, augmenting charge transfer resistance on both cathode and anode, particularly during fast charging or low temperatures (<10°C), promoting Na plating. Gas chromatography-mass spectrometry combined with density functional theory identified CO2 as the major gas generated from charged cathode/electrolyte interactions, exhibiting temperature/voltage dependence.
中文翻译:

高压钠离子电池弱溶剂化酯电解质
醋酸乙酯 (EA) 被认为是一种有前途的钠离子电池 (SIB) 电解质溶剂,具有低粘度、成本效益和低毒性。尽管分子动力学 (MD) 模拟揭示了大部分聚集与 Na+/EA 的弱溶剂化有关,但脉冲场梯度核磁共振 (pfg-NMR) 分析发现值得注意的 Na+ 扩散系数为 3.95×10− 10 m2 s−1,25°C,存在 1 m NaPF6 盐。采用氟代碳酸乙烯酯 (FEC) 作为成膜添加剂来形成电极-电解质界面,该电解质令人惊讶地制成了约 210 mAh Na0.97Ca0.03[Mn0.39Fe0.31Ni0.22Zn0.08]O2 (NCMFNZO)/硬碳 ( HC) 软包电池可实现 250 次循环的长循环寿命,容量保持率约为 80%,在 40°C 下循环至 4.0 V。 X 射线光电子能谱 (XPS) 显示,在循环过程中,相间有机物质不断增加,增加了阴极和阳极上的电荷转移电阻,特别是在快速充电或低温 (<10°C) 期间,促进了 Na 镀层。气相色谱-质谱法与密度泛函理论相结合,确定 CO2 是带电阴极/电解质相互作用产生的主要气体,表现出温度/电压依赖性。
更新日期:2024-07-05
中文翻译:

高压钠离子电池弱溶剂化酯电解质
醋酸乙酯 (EA) 被认为是一种有前途的钠离子电池 (SIB) 电解质溶剂,具有低粘度、成本效益和低毒性。尽管分子动力学 (MD) 模拟揭示了大部分聚集与 Na+/EA 的弱溶剂化有关,但脉冲场梯度核磁共振 (pfg-NMR) 分析发现值得注意的 Na+ 扩散系数为 3.95×10− 10 m2 s−1,25°C,存在 1 m NaPF6 盐。采用氟代碳酸乙烯酯 (FEC) 作为成膜添加剂来形成电极-电解质界面,该电解质令人惊讶地制成了约 210 mAh Na0.97Ca0.03[Mn0.39Fe0.31Ni0.22Zn0.08]O2 (NCMFNZO)/硬碳 ( HC) 软包电池可实现 250 次循环的长循环寿命,容量保持率约为 80%,在 40°C 下循环至 4.0 V。 X 射线光电子能谱 (XPS) 显示,在循环过程中,相间有机物质不断增加,增加了阴极和阳极上的电荷转移电阻,特别是在快速充电或低温 (<10°C) 期间,促进了 Na 镀层。气相色谱-质谱法与密度泛函理论相结合,确定 CO2 是带电阴极/电解质相互作用产生的主要气体,表现出温度/电压依赖性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号