当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced regioselective trifluoroalkylation of ketene dithioacetals with CF3SO2Na
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-17 , DOI: 10.1039/d4qo01203h Xiaoxiao Yang 1 , Xinyue Ma 1 , Pan Zhou 1 , Shuaiqi Lu 1 , Yongxin Zhang 1 , Chao Shu 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-17 , DOI: 10.1039/d4qo01203h Xiaoxiao Yang 1 , Xinyue Ma 1 , Pan Zhou 1 , Shuaiqi Lu 1 , Yongxin Zhang 1 , Chao Shu 1, 2
Affiliation
|
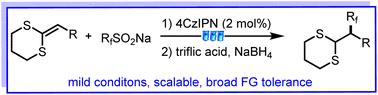
Fluoroorganic chemistry continues to be a fascinating tool for various areas including pharmaceuticals, agrochemicals, and materials. The incorporation of trifluoromethyl functional groups can yield remarkable changes in the chemical stability, metabolic stability and liposolubility of functional molecules. The development of efficient methods to synthesize trifluoromethyl compounds is of significant importance, and radical trifluoromethylation of alkenes is a highly attractive strategy for synthesizing trifluoromethylated compounds. In this study, regioselective hydrotrifluoromethylation of ketene dithioacetals with CF3SO2Na was achieved via a visible-light-promoted radical-polar process under mild and operationally simple conditions, using an inexpensive organic photocatalyst. The protocol features a low-cost catalyst, good functional group tolerance, a relatively broad substrate scope, and good to excellent yields. Gram-scale preparation and synthetic applications demonstrate opportunities to rapidly construct complex molecules. Mechanistic studies indicated that the transformation involves a regioselective radical addition/radical-polar crossover process.
中文翻译:

CF3SO2Na 光诱导烯酮二硫缩醛区域选择性三氟烷基化
含氟有机化学仍然是制药、农用化学品和材料等各个领域的一个令人着迷的工具。三氟甲基官能团的引入可以使功能分子的化学稳定性、代谢稳定性和脂溶性发生显着的变化。开发有效的三氟甲基化合物合成方法具有重要意义,而烯烃的自由基三氟甲基化是合成三氟甲基化化合物的一种极具吸引力的策略。在这项研究中,乙烯酮二硫缩醛与 CF 3 SO 2 Na 的区域选择性氢三氟甲基化是通过可见光促进的自由基极性过程在温和且操作简单的条件下实现的,使用廉价的有机光催化剂。该方案具有低成本的催化剂、良好的官能团耐受性、相对广泛的底物范围以及良好至优异的产率。克级制备和合成应用展示了快速构建复杂分子的机会。机理研究表明,该转变涉及区域选择性自由基加成/自由基-极性交叉过程。
更新日期:2024-07-17
中文翻译:

CF3SO2Na 光诱导烯酮二硫缩醛区域选择性三氟烷基化
含氟有机化学仍然是制药、农用化学品和材料等各个领域的一个令人着迷的工具。三氟甲基官能团的引入可以使功能分子的化学稳定性、代谢稳定性和脂溶性发生显着的变化。开发有效的三氟甲基化合物合成方法具有重要意义,而烯烃的自由基三氟甲基化是合成三氟甲基化化合物的一种极具吸引力的策略。在这项研究中,乙烯酮二硫缩醛与 CF 3 SO 2 Na 的区域选择性氢三氟甲基化是通过可见光促进的自由基极性过程在温和且操作简单的条件下实现的,使用廉价的有机光催化剂。该方案具有低成本的催化剂、良好的官能团耐受性、相对广泛的底物范围以及良好至优异的产率。克级制备和合成应用展示了快速构建复杂分子的机会。机理研究表明,该转变涉及区域选择性自由基加成/自由基-极性交叉过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号