Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Berberine analog of chloramphenicol exhibits a distinct mode of action and unveils ribosome plasticity
Structure ( IF 4.4 ) Pub Date : 2024-07-16 , DOI: 10.1016/j.str.2024.06.013 Zahra Batool 1 , Julia A Pavlova 2 , Madhura N Paranjpe 1 , Andrey G Tereshchenkov 2 , Dmitrii A Lukianov 3 , Ilya A Osterman 3 , Alexey A Bogdanov 4 , Natalia V Sumbatyan 3 , Yury S Polikanov 5
Structure ( IF 4.4 ) Pub Date : 2024-07-16 , DOI: 10.1016/j.str.2024.06.013 Zahra Batool 1 , Julia A Pavlova 2 , Madhura N Paranjpe 1 , Andrey G Tereshchenkov 2 , Dmitrii A Lukianov 3 , Ilya A Osterman 3 , Alexey A Bogdanov 4 , Natalia V Sumbatyan 3 , Yury S Polikanov 5
Affiliation
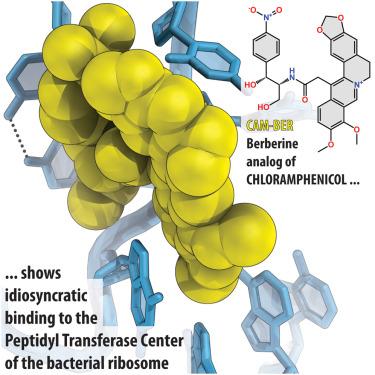
|
Chloramphenicol (CHL) is an antibiotic targeting the peptidyl transferase center in bacterial ribosomes. We synthesized a new analog, CAM-BER, by substituting the dichloroacetyl moiety of CHL with a positively charged aromatic berberine group. CAM-BER suppresses bacterial cell growth, inhibits protein synthesis in vitro , and binds tightly to the 70S ribosome. Crystal structure analysis reveals that the bulky berberine group folds into the P site of the peptidyl transferase center (PTC), where it competes with the formyl-methionine residue of the initiator tRNA. Our toe-printing data confirm that CAM-BER acts as a translation initiation inhibitor in stark contrast to CHL, a translation elongation inhibitor. Moreover, CAM-BER induces a distinct rearrangement of conformationally restrained nucleotide A2059, suggesting that the 23S rRNA plasticity is significantly higher than previously thought. CAM-BER shows potential in avoiding CHL resistance and presents opportunities for developing novel berberine derivatives of CHL through medicinal chemistry exploration.
中文翻译:

氯霉素的小檗碱类似物表现出独特的作用模式并揭示核糖体可塑性
氯霉素 (CHL) 是一种针对细菌核糖体中肽基转移酶中心的抗生素。我们通过用带正电荷的芳香小檗碱基团取代 CHL 的二氯乙酰基部分,合成了一种新的类似物 CAM-BER。 CAM-BER 抑制细菌细胞生长,抑制体外蛋白质合成,并与 70S 核糖体紧密结合。晶体结构分析表明,庞大的小檗碱基团折叠到肽基转移酶中心 (PTC) 的 P 位点,与起始 tRNA 的甲酰基蛋氨酸残基竞争。我们的脚趾打印数据证实,CAM-BER 作为翻译起始抑制剂,与 CHL(翻译延伸抑制剂)形成鲜明对比。此外,CAM-BER 诱导构象限制的核苷酸 A2059 发生明显的重排,表明 23S rRNA 的可塑性明显高于之前认为的。 CAM-BER 显示出避免 CHL 耐药性的潜力,并为通过药物化学探索开发新型 CHL 小檗碱衍生物提供了机会。
更新日期:2024-07-16
中文翻译:

氯霉素的小檗碱类似物表现出独特的作用模式并揭示核糖体可塑性
氯霉素 (CHL) 是一种针对细菌核糖体中肽基转移酶中心的抗生素。我们通过用带正电荷的芳香小檗碱基团取代 CHL 的二氯乙酰基部分,合成了一种新的类似物 CAM-BER。 CAM-BER 抑制细菌细胞生长,抑制体外蛋白质合成,并与 70S 核糖体紧密结合。晶体结构分析表明,庞大的小檗碱基团折叠到肽基转移酶中心 (PTC) 的 P 位点,与起始 tRNA 的甲酰基蛋氨酸残基竞争。我们的脚趾打印数据证实,CAM-BER 作为翻译起始抑制剂,与 CHL(翻译延伸抑制剂)形成鲜明对比。此外,CAM-BER 诱导构象限制的核苷酸 A2059 发生明显的重排,表明 23S rRNA 的可塑性明显高于之前认为的。 CAM-BER 显示出避免 CHL 耐药性的潜力,并为通过药物化学探索开发新型 CHL 小檗碱衍生物提供了机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号