当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper- and Palladium-Cocatalyzed Chemo-, Regio-, Stereo-, and Atroposelective Arylboration of 1,3-Enynes
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-15 , DOI: 10.1021/acscatal.4c03301 Wangyang Li 1 , Haohua Chen 2, 3 , Yanping Zheng 1 , Yong Lu 1 , Jinhui Xie 1 , Shanglin Chen 1 , Yu Lan 3, 4 , Qiuling Song 1, 5
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-07-15 , DOI: 10.1021/acscatal.4c03301 Wangyang Li 1 , Haohua Chen 2, 3 , Yanping Zheng 1 , Yong Lu 1 , Jinhui Xie 1 , Shanglin Chen 1 , Yu Lan 3, 4 , Qiuling Song 1, 5
Affiliation
|
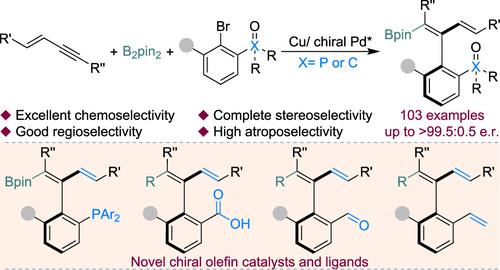
Catalytic enantioselective borylation reactions of unsaturated bonds as powerful tools for the synthesis of diverse chiral organoboron compounds have gained much attention and have wide applications in various fields. However, the atroposelective arylboration reaction with carbon–carbon triple bonds of 1,3-enynes to obtain axially chiral 1,3-dienylboronates remains an elusive and significant challenge. Hence, we develop a cooperative copper- and palladium-catalyzed arylboration reaction to assemble plentiful function enriched axially chiral 1,3-dienylboronates in a single step from easily available 1,3-enynes, B2pin2, and aryl bromides with high levels of chemo-, regio-, stereo-, and atroposelectivity. The mild reaction conditions lead to good functional group tolerance, which is proven by the broad substrate scope and late-stage functionalizations of bioactive compounds or drug molecules. Moreover, the reaction can be easily scaled up, and a series of further transformations can be achieved. It is worth emphasizing that several olefin catalysts and ligands with axial chirality can also be synthesized through the corresponding elaborations of such products, which further explains the powerful transformative ability and application potential of such axially chiral 1,3-dienylboronates. The mechanism experiment and density functional theory (DFT) calculations revealed the cooperative process of copper and palladium catalysis, indicating that the chemoselectivity and regioselectivity of boration are determined by the enyne insertion step on copper, and the atroposelectivity is controlled by the further reductive elimination on the palladium center. Meanwhile, the calculation also demonstrated that the distinct interactions between the P═O and C═O groups with the Pd or Bpin center in the key transition state lead to the formation of products with varying configurations while employing identical configuration ligands.
中文翻译:

铜和钯助催化 1,3-烯炔的化学、区域、立体和原子选择性芳基硼化
不饱和键的催化对映选择性硼化反应作为合成多种手性有机硼化合物的有力工具,引起了广泛的关注并在各个领域具有广泛的应用。然而,与 1,3-烯炔的碳-碳三键进行间质选择性芳基硼化反应以获得轴向手性 1,3-二烯基硼酸酯仍然是一个难以捉摸且重大的挑战。因此,我们开发了一种合作的铜和钯催化的芳基硼化反应,从容易获得的 1,3-烯炔,B 2 pin 2 和芳基溴化物具有高水平的化学选择性、区域选择性、立体选择性和阻容选择性。温和的反应条件导致良好的官能团耐受性,生物活性化合物或药物分子的广泛底物范围和后期官能化证明了这一点。此外,该反应可以轻松放大,并可以实现一系列进一步的转化。值得强调的是,通过此类产品的相应精制,还可以合成多种具有轴向手性的烯烃催化剂和配体,这进一步解释了此类轴向手性1,3-二烯基硼酸酯的强大转化能力和应用潜力。机理实验和密度泛函理论(DFT)计算揭示了铜和钯催化的协同过程,表明硼化的化学选择性和区域选择性是由铜上的烯炔插入步骤决定的,而去质选择性是由铜上的进一步还原消除控制的。钯中心。 同时,计算还表明,P=O和C=O基团与关键过渡态的Pd或Bpin中心之间的独特相互作用导致在采用相同构型配体的情况下形成不同构型的产物。
更新日期:2024-07-16
中文翻译:

铜和钯助催化 1,3-烯炔的化学、区域、立体和原子选择性芳基硼化
不饱和键的催化对映选择性硼化反应作为合成多种手性有机硼化合物的有力工具,引起了广泛的关注并在各个领域具有广泛的应用。然而,与 1,3-烯炔的碳-碳三键进行间质选择性芳基硼化反应以获得轴向手性 1,3-二烯基硼酸酯仍然是一个难以捉摸且重大的挑战。因此,我们开发了一种合作的铜和钯催化的芳基硼化反应,从容易获得的 1,3-烯炔,B 2 pin 2 和芳基溴化物具有高水平的化学选择性、区域选择性、立体选择性和阻容选择性。温和的反应条件导致良好的官能团耐受性,生物活性化合物或药物分子的广泛底物范围和后期官能化证明了这一点。此外,该反应可以轻松放大,并可以实现一系列进一步的转化。值得强调的是,通过此类产品的相应精制,还可以合成多种具有轴向手性的烯烃催化剂和配体,这进一步解释了此类轴向手性1,3-二烯基硼酸酯的强大转化能力和应用潜力。机理实验和密度泛函理论(DFT)计算揭示了铜和钯催化的协同过程,表明硼化的化学选择性和区域选择性是由铜上的烯炔插入步骤决定的,而去质选择性是由铜上的进一步还原消除控制的。钯中心。 同时,计算还表明,P=O和C=O基团与关键过渡态的Pd或Bpin中心之间的独特相互作用导致在采用相同构型配体的情况下形成不同构型的产物。












































 京公网安备 11010802027423号
京公网安备 11010802027423号