Molecular Diversity ( IF 3.9 ) Pub Date : 2024-07-16 , DOI: 10.1007/s11030-024-10934-5 Mohammad M Ibrahim 1 , Mohamad Nurul Azmi 2 , Maram B Alhawarri 3 , Nik Nur Syazni Nik Mohamed Kamal 4 , Hasan AbuMahmoud 1
|
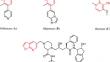
Pyridone heterocycles, such as furo[2,3-b]pyridines, have emerged as prominent scaffolds in medicinal chemistry due to their versatile pharmacological properties, including significant anticancer activity. In this study, we successfully synthesized new pyridine-2(H)-one, nicotinonitrile, and furo[2,3-b]pyridine derivatives from chalcones bearing 4-(benzyloxy)phenyl and dichlorothiophenyl subunits to explore their therapeutic potential against breast cancer. By employing a synthetic strategy involving Claisen-Schmidt condensation followed by sequential cyclizations and functional modifications, we synthesized and characterized four compounds (MI-S0, MI-S1, MI-S2, and MI-S3) using various spectroscopic methods, including FT-IR, 1H-NMR, 13C-NMR, DEPT, H,H- and C,H–COSY, and HRMS. The in vitro cytotoxic activity of these compounds was evaluated against two breast cancer cell lines, MCF-7 and MDA-MB-231, and compared with a noncancerous breast cell line, MCF-10A. All compounds exhibited potent cytotoxic activities with minimal selectivity toward normal cells. Molecular docking studies targeting the serine/threonine kinase AKT1, estrogen receptor alpha (ERα), and human epidermal growth factor receptor 2 (HER2) revealed strong binding affinities, suggesting a mechanism involving the disruption of key cellular signaling pathways. These findings underscore the potential of furo[2,3-b]pyridine derivatives as promising candidates for further development into anticancer agents, laying the groundwork for future investigations into their selective therapeutic efficacy and molecular mechanisms of action.
中文翻译:

新型吡啶-2(H)-酮、烟腈和呋喃[2,3-b]吡啶衍生物的合成、表征和生物活性
吡啶酮杂环化合物,例如呋喃并[2,3- b ]吡啶,由于其多种药理学特性(包括显着的抗癌活性)而成为药物化学领域的重要支架。在这项研究中,我们成功地从带有4-(苄氧基)苯基和二氯噻吩亚基的查耳酮中合成了新的吡啶-2( H )-酮、烟腈和呋喃[2,3- b ]吡啶衍生物,以探索它们对乳腺癌的治疗潜力。通过采用克莱森-施密特缩合、随后连续环化和功能修饰的合成策略,我们使用各种光谱方法(包括 FT-)合成并表征了四种化合物( MI-S0 、 MI-S1 、 MI-S2和MI-S3 )。 IR、 1 H-NMR、 13 C-NMR、DEPT、H,H-和 C,H-COSY 以及 HRMS。针对两种乳腺癌细胞系 MCF-7 和 MDA-MB-231 评估了这些化合物的体外细胞毒活性,并与非癌性乳腺癌细胞系 MCF-10A 进行了比较。所有化合物都表现出有效的细胞毒活性,对正常细胞的选择性极低。针对丝氨酸/苏氨酸激酶 AKT1、雌激素受体 α (ERα) 和人表皮生长因子受体 2 (HER2) 的分子对接研究揭示了很强的结合亲和力,表明涉及破坏关键细胞信号传导途径的机制。 这些发现强调了呋喃并[2,3- b ]吡啶衍生物作为进一步开发抗癌药物的有希望的候选者的潜力,为未来研究其选择性治疗功效和分子作用机制奠定了基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号